Please contribute to this project, if you have more information about this term feel free to edit this page.
Sexual selection
From Wikipedia, the free encyclopedia
This article is about the evolutionary concept. For the selection of the sex of offspring, see
sex selection.
Sexual selection is a mode of
natural selection in which some individuals out-reproduce others of a population because they are better at securing
mates for
sexual reproduction.
[1][2]
Sexual selection can lead organisms to extreme efforts in order to
reproduce, such as combat and display, or produce extreme features.
Examples of features influenced by sexual selection include ornate
peacock feathers,
birds of paradise, the antlers of
stags (male deer), and the manes of lions.
The concept of sexual selection was first articulated by
Charles Darwin
in the 19th century. His ideas on sexual selection were met with
scepticism by his contemporaries and not considered of great importance
in the 20th century, so that in the 1930s biologists decided to include
sexual selection as a mode of natural selection.
[3] Only in the 21st Century have they become more important in
biology.
[4] He also speculated on
sexual selection in human evolution.
[2]
Darwin's ideas
In 1858,
[5]
Darwin described sexual selection as an important process driving
species evolution and as a significant element of his theory of natural
selection, but this concept was only named in his 1859 book
On the Origin of Species. The sexual form of selection
... depends, not on a struggle for existence, but on a struggle
between the males for possession of the females; the result is not death
to the unsuccessful competitor, but few or no offspring.[6]
... when the males and females of any animal have the same general
habits ... but differ in structure, colour, or ornament, such
differences have been mainly caused by sexual selection.[7]
Darwin greatly expanded his initial three-page treatment of sexual selection in the 1871 book
The Descent of Man and Selection in Relation to Sex.
This 900-page, two-volume work includes 70 pages on sexual selection in
human evolution, and 500 pages on sexual selection in other animals.
[8]
In summary, while natural selection results from the struggle to
survive, sexual selection emerges from the struggle to reproduce.
The sexual struggle is of two kinds; in the one it is between
individuals of the same sex, generally the males, in order to drive away
or kill their rivals, the females remaining passive; whilst in the
other, the struggle is likewise between the individuals of the same sex,
in order to excite or charm those of the opposite sex, generally the
females, which no longer remain passive, but select the more agreeable
partners.[9]
Concept
The concept of sexual selection arose from the observation that many
animals had evolved features whose function was not to help those
individuals survive (and indeed, which might be deleterious to their
individual survival), but to help them to maximize their
reproductive success. This can be realized in two different ways:
- by making themselves attractive to the opposite sex (intersexual selection, between the sexes), or
- by intimidating, deterring or defeating same-sex rivals (intrasexual selection, within a given sex).
Thus, sexual selection takes two major forms:
intersexual selection (also known as '
mate choice' or 'female choice') in which males compete with each other to be chosen by females; and
intrasexual selection
(also known as 'male–male competition') in which members of the less
limited sex (typically males) compete aggressively among themselves for
access to the limiting sex. The
limiting sex is the sex which has the higher parental investment, which therefore faces the most pressure to make a good mate decision.
Extinct
Irish elk (
Megaloceros giganteus). These antlers span 2.7 metres (8.9 ft) and have a mass of 40 kg (88 lb).
For
intersexual selection to work, one sex must evolve a
feature alluring to the opposite sex, sometimes resulting in a "fashion
fad" of intense selection in an arbitrary direction. Or, in the second
case, while natural selection can help animals develop ways of killing
or escaping from other
species,
intrasexual selection drives the selection of attributes that allow
alpha males to dominate their own breeding partners and rivals.
[10]
Sexual selection sometimes generates monstrously absurd features
that, in harder times, may help cause a species' extinction, as has been
suggested
[8] for the giant antlers of the Irish elk (
Megaloceros giganteus) that became extinct in
Pleistocene Europe.
[11] However, sexual selection can also do the opposite, driving species divergence - sometimes through elaborate changes in
genitalia - such that new species emerge.
[12]
Although the driving force for both sexes is reproductive success,
the two genders have different ways to maximize it: males benefit from
frequent mating, including by monopolizing access to a group of fertile
females, because new matings make them increase the number of eggs they
fertilize. Females have a limited number of offspring they can have
which is not increased by mating more frequently. They maximize the
return on the energy they invest in reproduction by seeing their
offspring grow into healthy adults, such as sons with well-developed,
sexually attractive features, which
sire many descendants, or fecund daughters. In addition, males often invest less of their energy in each individual offspring
[6]
whereas female energy expenditures on gestation and parental care being
much higher. Females have much more reason to be "picky". They need a
way to choose males that are most likely to produce high-quality
offspring.
Modern Interpretation
Today, biologists would say that certain evolutionary traits can be explained by
intraspecific competition - competition between members of the same species - distinguishing between competition
before or
after sexual intercourse.
- Before copulation, intrasexual selection - usually between males - may take the form of male-to-male combat. Also, intersexual selection, or mate choice, occurs when females choose between male mates.[13]
Traits selected by male combat are called secondary sexual
characteristics (including horns, antlers, etc.), which Darwin described
as "weapons", while traits selected by mate (usually female) choice are
called "ornaments".
- After copulation, male–male competition distinct from conventional aggression may take the form of sperm competition, as described by Parker[14] in 1970. More recently, interest has arisen in cryptic female choice,[15] a phenomenon of internally fertilised animals such as mammals and birds, where a female will get rid of a male's sperm without his knowledge.
Finally,
sexual conflict is said to occur between breeding partners,
[16] sometimes leading to an
evolutionary arms race between males and females.
Female mating preferences are widely recognized as being responsible
for the rapid and divergent evolution of male secondary sexual traits.
[17]
Females of many animal species prefer to mate with males with external
ornaments - exaggerated features of morphology such as elaborate sex
organs. These preferences may arise when an arbitrary female preference
for some aspect of male morphology — initially, perhaps, a result of
genetic drift — creates, in due course, selection for males with the appropriate ornament. One interpretation of this is known as the
sexy son hypothesis. Alternatively, genes that enable males to develop impressive ornaments or fighting ability may simply show off greater
disease resistance or a more efficient
metabolism, features that also benefit females. This idea is known as the
good genes hypothesis.
Criteria for reproductive success
The success of an organism is not only measured by the number of
offspring left behind, but by the quality or probable fitness of the
offspring: their
reproductive fitness. Sexual selection increases the ability of organisms to differentiate one another at the
species level: inter
species selection.
The grossest blunder in sexual preference, which we can conceive of
an animal making, would be to mate with a species different from its
own, and with which hybrids are either infertile, or, through the
mixture of instincts and other attributes appropriate to different
courses of life, at so serious a disadvantage as to leave no
descendants. ... it is no conjecture that a discriminative mechanism
exists, variations in which will be capable of giving rise to a similar
discrimination within its own species, should such a discrimination
become at any time advantageous.
Individuals in each region most readily attracted to, or excited by,
mates of the type there favoured, in contrast to possible mates of the
opposite type, will, in fact, be the better represented in future
generations, and both the discrimination and the preference will thereby
be enhanced. It appears certainly possible that an evolution of sexual
preference due to this cause would establish an effective isolation
between two differentiated parts of a species, even when geographical
and other factors were least favourable to such separation.
Ronald Fisher, 1930
The expansion of interspecies selection and intraspecies selection is
a driving force behind species fission: the separation of a single
contiguous species into multiple non-contiguous variants. Sexual
preference creates a tendency towards
assortative mating or
homogamy,
providing a system by which a group otherwise invaded by diverse genes
is able to suppress their effects and diverge genetically.
The general conditions of sexual discrimination appear to be (1) the
acceptance of one mate precludes the effective acceptance of alternative
mates, and (2) the rejection of an offer will be followed by other
offers, either certainly, or at such high chance that the risk of
non-occurrence will be smaller than the chance advantage to be gained by
selecting a mate.
Example: intersexual selection
Main article:
Mate choice
The conditions determining which sex becomes the more limited
resource in intersexual selection can be best understood by way of
Bateman's principle which states that
the sex which invests the most in producing offspring becomes a limiting resource over which the other sex will compete. This can be most easily illustrated by the contrast in nutritional investment into a
zygote
between egg and sperm, and the limited reproductive capacity of females
compared to males. Thus, 'sexual selection' typically refers to the
process of choice (the limiting factor, which is typically females) over
members of the opposite sex (the non-limited factor, typically males).
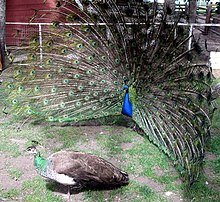
The
peacock
provides a particularly well known example of intersexual selection,
where ornate males compete to be chosen by females. The result is a
stunning feathered display, which is large and unwieldy enough to pose a
significant survival disadvantage. Biologists have suggested that the
layers of the ornate plumage of males provide a means of demonstrating
body symmetry, such that peahens are "trying" to discover the health of
the male or the
quality of his genes. Diseases, injuries, and genetic disorders may impair the body's symmetry.
Bird species often demonstrate intersexual selection, perhaps because
- due to their lightweight body structures - fights between males may
be ineffective or impractical. Therefore, male birds commonly use the
following methods to try to seduce the females:
- Colour: Some species have ornate, diverse, and often colourful feathers.
- Song: Male birdsong provides an important way of protecting territory (intrasexual selection).
- Nest construction: In some species, males build nests that females
subject to rigorous inspection, choosing the male that makes the most
attractive nest.
- Dance: Males dance in front of females. Cranes provide a well-known example.[10]
As a propagandist, the cock behaves as though he knew that it was as
advantageous to impress the males as the females of his species, and a
sprightly bearing with fine feathers and triumphant song are quite as
well adapted for war-propaganda as for courtship. —Ronald Fisher, 1930
In some bird species, both the male and the female contribute a great
deal to offspring-care. In these cases, the male and female will be
continuously assessing each other based on sexual characteristics. In
the
blue-footed booby,
the females tend to choose males with brighter blue feet, because birds
with brighter feet are younger, and thus have greater fertility and
ability to provide paternal care.
[18]
When researchers put make-up on the males' feet to make them look
duller after the laying of the first eggs, their mates consequently laid
smaller second eggs, which shows that female boobies continuously
evaluate their mates' reproductive value.
[18]
Males also vary their behaviour based on the females' foot colour.
Males mated to females with brighter feet are more willing to incubate
their eggs.
[19]
Exponential growth in female preference
In species where intersexual selection is active, as in many
polygamous birds, sexual selection acts by accelerating the preference
that specific "fashion" ornaments attract, causing the preferred trait
and
female preference for it to increase together, explosively. While
Darwin had been criticised for simply accepting female whims as given,
Ronald Fisher grasped the underlying mechanism in
The Genetical Theory of Natural Selection, in a remark that was not widely understood
[20] for another 50 years:
... plumage development in the male, and sexual preference for such
developments in the female, must thus advance together, and so long as
the process is unchecked by severe counterselection, will advance with
ever-increasing speed. In the total absence of such checks, it is easy
to see that the speed of development will be proportional to the
development already attained, which will therefore increase with time exponentially, or in geometric progression. —Ronald Fisher, 1930
Fisher's runaway process
causes a dramatic increase in both the male's conspicuous feature and
in female preference for it, until practical, physical constraints halt
further exaggeration. A
positive feedback
loop is created, producing extravagant physical structures in the
non-limiting sex. A classic example of female choice and potential
runaway selection is the
long-tailed widowbird
(left). While males have long tails that are selected for by female
choice, female tastes in tail length are still more extreme with females
being attracted to tails longer than those that naturally occur.
[21]
Fisher understood that female preference for long tails may be passed
on genetically, in conjunction with genes for the long tail itself.
Long-tailed widowbird offspring of both sexes will inherit both sets of
genes, with females
expressing their genetic preference for long tails, and males showing off the coveted long tail itself.
[20]
Richard Dawkins presents a non-mathematical explanation of the runaway sexual selection process in his book
The Blind Watchmaker.
[20]
Females who prefer long tailed males tend to have mothers that chose
long-tailed fathers. As a result, they carry both sets of genes in their
bodies. That is, genes for long tails and for preferring long tails
become
linked.
The taste for long tails and tail length itself may therefore become
correlated, tending to increase together. The more tails lengthen, the
more long tails are desired. Any slight initial imbalance between taste
and tails may set off an explosion in tail lengths. Fisher corresponded
that:
The exponential element, which is the kernel of the thing, arises
from the rate of change in hen taste being proportional to the absolute
average degree of taste. —Ronald Fisher, 1932[22]
The female widow bird will desire to mate with the most attractive
long-tailed male so that her progeny, if male, will themselves be
attractive to females of the next generation - thereby fathering many
offspring who will carry the female's genes. Since the rate of change in
preference is proportional to the average taste amongst females, and as
females desire to secure the services of the most sexually attractive
males, an additive effect is created that, if unchecked, can yield
exponential increases in a given taste and in the corresponding desired
sexual attribute.
It is important to notice that the conditions of relative stability
brought about by these or other means, will be far longer duration than
the process in which the ornaments are evolved. In most existing species
the runaway process must have been already checked, and we should
expect that the more extraordinary developments of sexual plumage are
not due like most characters to a long and even course of evolutionary
progress, but to sudden spurts of change. —Ronald Fisher, 1930
Since Fisher's initial conceptual model of the 'runaway' process, Russell Lande
[23] and Peter O'Donald
[24] have provided detailed mathematical proofs that define the circumstances under which runaway sexual selection can take place.
Example: intrasexual selection
A good example of intrasexual selection, in which males fight for dominance over a
harem of females, is the
elephant seal - large, oceangoing mammals of the
genus Mirounga. There are two species: the
northern (
M. angustirostris) and
southern elephant seal (
M. leonina) - the largest
carnivore living today. Both species show extreme
sexual dimorphism, possibly the largest of any mammal, with southern males typically five to six times heavier than the females.
[25]
While the females average 400 to 900 kilograms (880 to 1,980 lb) and
2.6 to 3 metres (8.5 to 9.8 ft) long, the bulls average 2,200 to 4,000
kilograms (4,900 to 8,800 lb) and 4.2 to 5 metres (14 to 16 ft) long.
[26][27] The record-sized bull, shot in Possession Bay,
South Georgia, on February 28, 1913, measured 6.85 metres (22.5 ft) long and was estimated to weigh 5,000 kilograms (11,000 lb).
[28][29] The maximum weight of a female is 1,000 kilograms (2,200 lb) with a length of 3.7 metres (12 ft).
Males arrive in the colonies before the females and fight for control of harems.
[30] Large body size confers advantages in fighting. The
agonistic behaviour of the bulls gives rise to a dominance hierarchy, with access to harems and breeding activity being determined by rank.
[31] The dominant bulls or "harem masters" establish
harems
of several dozen females. The least successful males have no harems,
but may try to copulate with a harem male's females when the dominant
male is not looking. A dominant male must stay in his territory to
defend it, which can mean months without eating, living on his store of
blubber. Some males have stayed ashore for more than three months
without food. Two fighting males use their weight and canine teeth
against each other. The outcome is rarely fatal, and the defeated bull
will flee; however, bulls suffer severe tears and cuts. Males commonly
vocalize with a coughing roar that serves in both individual recognition
and size assessment. Conflicts between high-ranking males are more
often resolved with posturing and vocalizing than with physical contact.
[31]
Male
northern elephant seals fight fiercely each year. Unsuccessful males may not mate at all, while successful males have harems of 30 to 100 females.
In the case of
intrasexual selection, adorned males may gain a
reproductive advantage without the intervention of female preference.
This advantage will be conferred by weapons used in the process of
resolving disputes, such as those over territorial rights. The use of
sexual ornamentation as a
signaling device to create a
dominance hierarchy among males, also known as a
pecking order,
allows struggle to proceed without excessive injury or fatality. It is
predominantly when two opposing males are so closely matched, as would
be found in males not having established themselves in a dominance
hierarchy, that asymmetries cannot be found and the confrontation
escalates to a point where the asymmetries must be proved by aggressive
use of ornamentation.
How often males will physically engage each other, and in what manner, can best be understood by applying
game theory developed for biology, most notably by
John Maynard Smith.
[32]
Sexual dimorphism
Sex differences directly related to reproduction and serving no direct purpose in
courtship are called
primary sexual characteristics.
Traits amenable to sexual selection, which give an organism an
advantage over its rivals (such as in courtship) without being directly
involved in
reproduction, are called
secondary sex characteristics.
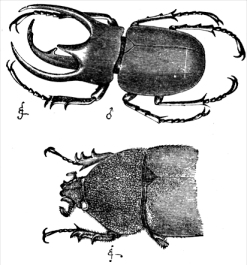
The
rhinoceros beetle demonstrates a classic case of sexual dimorphism. This plate is from Darwin's
Descent of Man, with the male at top, female at bottom.
In most sexual species the
males and
females have different
equilibrium
strategies, due to a difference in relative investment in producing
offspring. As formulated in Bateman's principle, females have a greater
initial investment in producing offspring (
pregnancy in mammals or the production of the egg in
birds and
reptiles),
and this difference in initial investment creates differences in
variance in expected reproductive success and bootstraps the sexual
selection processes. Classic examples of reversed sex-role species
include the
pipefish, and Wilson's phalarope. Also, unlike a female, a male (except in
monogamous
species) has some uncertainty about whether or not he is the true
parent of a child, and so will be less interested in spending his energy
helping to raise offspring that may or may not be related to him. As a
result of these factors, males are typically more willing to mate than
females, and so females are typically the ones doing the choosing
(except in cases of
forced copulations, which can occur in certain species of
primates,
ducks, and others). The effects of sexual selection are thus held to typically be more pronounced in males than in females.
Differences in secondary sexual characteristics between males and females of a species are referred to as
sexual dimorphisms.
These can be as subtle as a size difference (sexual size dimorphism,
often abbreviated as SSD) or as extreme as horns and colour patterns.
Sexual dimorphisms abound in nature. Examples include the possession of
antlers by only male
deer,
the brighter coloration of many male birds in comparison with females
of the same species, or even more distinct differences in basic
morphology, such as the drastically increased eye-span of the male
stalk-eyed fly. The
peacock, with its elaborate and colourful tail feathers, which the
peahen lacks, is often referred to as perhaps the most extraordinary example of a dimorphism. Male and female
black-throated blue warblers and
Guianan cock-of-the-rocks
also differ radically in their plumage. Early naturalists even believed
the females to be a separate species. The largest sexual size
dimorphism in
vertebrates is the
shell dwelling cichlid fish Neolamprologus callipterus in which males are up to 30 times the size of females. Many other fish such as
guppies also exhibit sexual dimorphism. Extreme sexual size dimorphism, with females larger than males, is quite common in
spiders.
Sexual selection as a toolkit of natural selection
Sexual selection may explain how certain characteristics (such as
feathers) had distinct survival value at an early stage in their
evolution.
[10]
One recent theory sees evolution as an "adventure quest" in which
species develop complexity and novelty by acquiring modular capabilities
through chance encounters in an evolutionary game.
[33] But this still leaves open the question of how natural selection initiated each module.
Geoffrey Miller proposes that sexual selection might have contributed by creating evolutionary modules such as
Archaeopteryx feathers as sexual ornaments, at first. The earliest proto-birds such as China's
Protarchaeopteryx,
discovered in the early 1990s, had well-developed feathers but no sign
of the top/bottom asymmetry that gives wings lift. Some have suggested
that the feathers served as insulation, helping females incubate their
eggs. But perhaps the feathers served as the kinds of sexual ornaments
still common in most bird species, and especially in birds such as
peacocks and birds-of-paradise today. If proto-bird courtship displays
combined displays of forelimb feathers with energetic jumps, then the
transition from display to aerodynamic functions could have been
relatively smooth.
[8]
Viability and variations of the theory
Due to their sometimes greatly exaggerated nature, secondary sexual
characteristics can prove to be a hindrance to an animal, thereby
lowering its chances of survival. For example, the large antlers of a
moose are bulky and heavy and slow the creature's flight from predators;
they also can become entangled in low-hanging tree branches and shrubs,
and undoubtedly have led to the demise of many individuals. Bright
colourations and showy ornamenations, such as those seen in many male
birds, in addition to capturing the eyes of females, also attract the
attention of predators. Some of these traits also represent
energetically costly investments for the animals that bear them. Because
traits held to be due to sexual selection often conflict with the
survival fitness of the individual, the question then arises as to why,
in nature, in which
survival of the fittest is considered the rule of thumb, such apparent liabilities are allowed to persist.
An often-cited theory published by
R.A. Fisher in 1930 that attempts to resolve the paradox posits that such traits are the results of explosive
positive feedback
loops that have as their starting points particular sexual preferences
for features that confer a survival advantage and thus "become
established in the species." Fisher argued that such features advance in
the direction of the preference even beyond the optimal level for
survival, until the selection pressure of female choice is precisely
counterbalanced by the resultant disadvantage for survival. Fisher
further argued that the strength of the female preference tends to grow
exponentially (leading to 'explosive' evolution of the characteristic)
until finally checked by ecological selection, since the offspring of
those females with the strongest preference typically fare better in
reproducing than the offspring of females with weaker preferences. Any
mutations for the preference opposite to the given characteristic,
though tending to promote survival against ecological selection,
nevertheless tend not to survive in the
gene pool because male offspring that result from
matings
based on the preference are less sexually attractive to the majority of
the females in the population, and thus infrequently chosen as mates.
An equivalent way of expressing this is that if most females are
looking, for example, for long-tailed males, then each female
individually does better to select a long-tailed male, since then her
male children are more likely to succeed. (The females do not actually
have this thought process; this kind of "decision" is an
evolutionarily stable strategy.)
Other theories highlight intrinsically useful qualities of such
traits. Antlers, horns and the like can be used in physical defence from
a
predator, and also in competition among males in a
tournament species.
The winner, which typically becomes the dominant animal in the
population, is granted access to females, and therefore increases his
reproductive output. Antlers are not the only mechanism that can be used
to counteract predation. Predators typically look for the eyes of their
prey so they can attack that end of the creature. The conspicuousness
of eyespots on many species of butterflies and fishes confuses predators
and helps to prevent the prey from suffering serious damage.
[34]
Another, more recently developed, theory, the
handicap principle of
Amotz Zahavi,
Russell Lande and
W. D. Hamilton,
holds that the fact that the male of the species is able to survive
until and through the age of reproduction with such a seemingly
maladaptive trait is effectively considered by the female to be a
testament to his overall fitness. Such handicaps might prove he is
either free of or resistant to
disease,
or it might demonstrate that this animal possesses more speed or a
greater physical strength that is used to combat the troubles brought on
by the exaggerated trait.
Zahavi's work spurred a re-examination of the field, which has
produced an ever-accelerating number of theories. In 1984, Hamilton and
Marlene Zuk
introduced the "Bright Male" hypothesis, suggesting that male
elaborations might serve as a marker of health, by exaggerating the
effects of disease and deficiency. In 1990, Michael Ryan and A.S. Rand,
working with the túngara frog, proposed the hypothesis of "Sensory
Exploitation", where exaggerated male traits may provide a sensory
stimulation that females find hard to resist. Subsequently the theories
of the "Gravity Hypothesis" by Jordi Moya-Larano et al. and "Chase Away"
by Brett Holland and William R. Rice have also been added. In addition,
in the late 1970s Janzen and Mary Willson, noting that male flowers are
often larger than female flowers, expanded the field of sexual
selection into
plants.
In the past few years, the field has exploded to include many
additional observations and areas of study, not all of which are clearly
included under Darwin's definition of sexual selection. These include
cuckoldry, nuptial gifts,
sperm competition,
infanticide, physical
beauty,
mating by subterfuge, species isolation mechanisms, male parental care,
ambiparental care, mate location, polygamy, and mechanisms that can
only be called bizarre, including homosexual rape in certain male
animals, cementing of females' vaginal pores by males in some
lepidopteran insects, and
insect penises
specialized to remove any sperm packets from females which may have
been deposited by previous suitors. These theories are not mutually
exclusive; combinations of them may also be considered.
Focusing on the effect of
sexual conflict, as hypothesized by William Rice, Locke Rowe et Göran Arnvist,
Thierry Lodé[35] underlines that the divergence of interest constitutes a key for evolutionary process. Sexual conflict leads to an
antagonistic co-evolution in which one sex tends to control the other, resulting in a tug of war. Besides,
the sexual propaganda theory
only argued that mate were opportunistically lead, on the basis of
various factors determining the choice such as phenotypic
characteristics, apparent vigour of individual, strength of mate
signals, trophic resources, territoriality etc. and could explain the
maintenance of genetic diversity within populations.
Several workers have brought attention to the fact that elaborated
characters that ought to be costly in one way or another for their
bearers (e.g., the
tails of some species of
Xiphophorus
fish) do not always appear to have a cost in terms of energetics,
performance or even survival. One possible explanation for the apparent
lack of costs is that "compensatory traits" have evolved in concert with
the sexually selected traits.
[36]
In humans
Charles Darwin conjectured that the male beard was the product of sexual selection in prehistoric times
Charles Darwin conjectured that the male beard, as well as the
relative hairlessness of humans compared to nearly all other mammals,
are results of sexual selection. He reasoned that since, compared to
males, the bodies of females are more nearly hairless, hairlessness is
one of the atypical cases due to its selection by males at a remote
prehistoric time, when males had overwhelming selective power, and that
it nonetheless affected males due to genetic correlation between the
sexes. He also hypothesized that contrasts in sexual selection acting
along with natural selection were significant in the geographical
differentiation in human appearance of some isolated groups as he did
not believe that natural selection alone provided a satisfactory answer.
As an example for this, he implicitly mentions
[clarification needed] Steatopygia in
Khoisan women.
[37]
Geoffrey Miller,
drawing on some of Darwin's largely neglected ideas about human
behaviour, has hypothesized that many human behaviours not clearly tied
to survival benefits, such as humour, music, visual art, verbal
creativity, and some forms of altruism, are courtship adaptations that
have been favoured through sexual selection. In that view, many human
artefacts could be considered subject to sexual selection as part of the
extended phenotype,
for instance clothing that enhances sexually selected traits. The
German anthropologist Ferdinand Fellman argues that the emergence of
human self-consciousness is due to an extended sexual selection, termed
"emotional selection", bridging the gap between
animal sexual behaviour and human erotic love.
[38]
Some hypotheses about the evolution of the human brain argue that it
is a sexually selected trait, as it would not confer enough fitness in
itself relative to its high maintenance costs (a quarter to a fifth of
the energy and oxygen consumed by a human).
[39]
Related to this is vocabulary, where humans, on average, know far more
words than are necessary for communication. Miller (2000) has proposed
that this apparent redundancy is due to individuals using vocabulary to
demonstrate their intelligence, and consequently their "fitness", to
potential mates. This has been tested experimentally and it appears that
males do make greater use of lower frequency (more unusual) words when
in a romantic mindset compared to a non-romantic mindset, meaning that
vocabulary is likely to be used as a sexual display.
[40]
An uncertain example: the giraffe
The evolutionary origins of the
giraffe's (
Giraffa camelopardalis) long neck are controversial. The long-accepted "competing browser's hypothesis" originally put forth by
Charles Darwin has been put into question. Originally, scientists believed that the elongation of the giraffe's neck had been a result of
natural selection acting in relation to
foraging behaviour, where it was supposed that longer necks enabled favoured individuals to gather food inaccessible to other animals.
[41] But even though the giraffe’s overall height is about 6 meters, it still typically feeds at about 2 meters above the ground.
[42]
Moreover, the giraffe's kudu, impala, and steenbok competitors do not
feed above 2 meters and prefer feeding at shoulder level as well, rather
than at the maximum height they could reach.
[43]
An alternative explanation for the origin of long necks in giraffe is sexual selection. Male giraffe often
neck with other males to exhibit dominance.
[44]
There are six criteria that need to be satisfied for the exaggerated
neck to be classified as a result of sexual selection. The
characteristic should be more exaggerated in one of the sexes; it must
be used to indicate dominance; have no direct survival benefits; cost
the organism in terms of survival or other factors (e.g., energetics
[45][46]); positive
allometry should be observed.
[47]
But evolutionary history shows that increased neck length is not
correlated to increases in other parts of the body, which would be
expected from foraging selection, so sexual selection may be a more
satisfactory explanation.
[48] Studies have failed to resolve the causes involved: perhaps the neck was a result of both or other forces.
[49]
History and application of the theory
The theory of sexual selection was first proposed by Charles Darwin in his book
The Origin of Species, though it was primarily devoted to natural selection. A later work,
The Descent of Man and Selection in Relation to Sex
dealt with the subject of sexual selection exhaustively, in part
because Darwin felt that natural selection alone was unable to account
for certain types of apparently non-competitive adaptations, such as the
tail of a male
peacock.
He once wrote to a colleague that "The sight of a feather in a
peacock's tail, whenever I gaze at it, makes me sick!" His work divided
sexual selection into two primary categories: male-male competition
(which would produce adaptations such as a
bighorn sheep's
horns, which are used primarily in sparring with other males over
females), and cases of female choice (which would produce adaptations
like beautiful plumage, elaborate songs, and other things related to
impressing and attracting).
Darwin's views on sexual selection were opposed strongly by his "co-discoverer" of natural selection,
Alfred Russel Wallace,
though much of his "debate" with Darwin took place after Darwin's
death. Wallace argued that the aspects of it which were male-male
competition, while real, were simply forms of natural selection, and
that the notion of "female choice" was attributing the ability to judge
standards of beauty to animals far too cognitively undeveloped to be
capable of aesthetic feeling (such as
beetles).
Wallace also argued that Darwin too much favoured the bright colours
of the male peacock as adaptive without realizing that the "drab"
peahen's coloration is itself adaptive, as
camouflage. Wallace more speculatively argued that the bright colours and long tails of the peacock were
not
adaptive in any way, and that bright colouration could result from
non-adaptive physiological development (for example, the internal organs
of animals, not being subject to a visual form of natural selection,
come in a wide variety of bright colours). This has been questioned by
later scholars as quite a stretch for Wallace, who in this particular
instance abandoned his normally strict "
adaptationist"
agenda in asserting that the highly intricate and developed forms such
as a peacock's tail resulted by sheer "physiological processes" that
were somehow not at all subjected to adaptation.
Though Darwin considered sexual and natural selection to be two
separate processes of equal importance, most of his contemporaries were
not convinced, and sexual selection is usually de-emphasized as being a
lesser force than, or simply a part of, natural selection.
The sciences of
evolutionary psychology,
human behavioural ecology, and
sociobiology study the influence of sexual selection in humans, though these are often controversial fields.
See also
Notes
 The rhinoceros beetle demonstrates a classic case of sexual dimorphism. This plate is from Darwin's Descent of Man, with the male at top, female at bottom.
The rhinoceros beetle demonstrates a classic case of sexual dimorphism. This plate is from Darwin's Descent of Man, with the male at top, female at bottom.









No comments:
Post a Comment