begin quote from:
Permian–Triassic extinction event
Permian–Triassic extinction event
From Wikipedia, the free encyclopedia
%
Millions of years ago
P–Tr
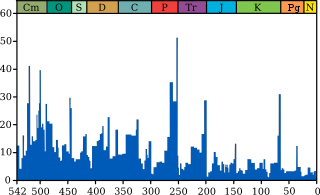
Plot of extinction intensity (percentage of
genera that are present in each interval of time but do not exist in the following interval) vs time in the past for marine genera.
[1]
Geological periods are annotated (by abbreviation and colour) above.
The Permian–Triassic extinction event is the most significant event for
marine genera, with just over 50% (according to this source) failing to
survive.
(source and image info)
The
Permian–Triassic (
P–Tr)
extinction event, colloquially known as the
Great Dying, the
End Permian or the
Great Permian Extinction,
[2][3] occurred about 252
Ma (million years) ago,
[4] forming the boundary between the
Permian and
Triassic geologic periods, as well as the
Paleozoic and
Mesozoic eras. It is the Earth's most severe known
extinction event, with up to 96% of all
marine species[5][6] and 70% of
terrestrial vertebrate species becoming
extinct.
[7] It is the only known mass extinction of
insects.
[8][9] Some 57% of all
families and 83% of all
genera became extinct. Because so much
biodiversity was lost, the recovery of life on Earth took significantly longer than after any other extinction event,
[5] possibly up to 10 million years.
[10]
There is evidence for between one and three distinct pulses, or phases, of extinction.
[7][11][12][13] Suggested mechanisms for the latter include one or more large
bolide impact events, massive
volcanism, coal or gas fires and explosions from the
Siberian Traps,
[14] and a runaway
greenhouse effect triggered by sudden release of
methane from the sea floor due to
methane clathrate dissociation or
methane-producing microbes known as methanogens;
[15] possible contributing gradual changes include sea-level change, increasing
anoxia, increasing
aridity, and a shift in ocean circulation driven by
climate change.
Dating the extinction
Permian–Triassic extinction
-262 —
–
-260 —
–
-258 —
–
-256 —
–
-254 —
–
-252 —
–
-250 —
–
-248 —
–
-246 —
–
-244 —
–
-242 —
–
-240 —
–
-238 —
←
δ13C stabilises at +2 ‰. Calcified green algae return.[16]
←
Full recovery of woody trees[18]
An approximate timescale of events around the P-Tr boundary.
Axis scale: millions of years ago.
Until 2000, it was thought that rock sequences spanning the
Permian–Triassic boundary were too few and contained too many gaps for
scientists to reliably determine its details.
[20]
However, it is now possible to date the extinction with remarkable
precision. U-Pb zircon dates from five volcanic ash beds from the Global
Stratotype Section and Point for the Permian-Triassic boundary at
Meishan, China, establish a high-resolution age model for the extinction
– allowing exploration of the links between global environmental
perturbation, carbon cycle disruption, mass extinction, and recovery at
millennial timescales. The extinction occurred between 251.941 ± 0.037
and 251.880 ± 0.031 Ma, a duration of 60 ± 48 ka.
[21] A large (approximately 0.9%), abrupt global decrease in the
ratio of the stable isotope
13C to that of
12C, coincides with this extinction,
[18][22][23][24][25] and is sometimes used to identify the Permian–Triassic boundary in rocks that are unsuitable for radiometric dating.
[26] Further evidence for environmental change around the P–Tr boundary suggests an 8 °C (14 °F) rise in temperature,
[18] and an increase in
CO
2 levels by
2000 ppm (by contrast, the concentration immediately before the industrial revolution was
280 ppm.)
[18] There is also evidence of increased ultraviolet radiation reaching the earth causing the mutation of plant spores.
[18]
It has been suggested that the Permian–Triassic boundary is
associated with a sharp increase in the abundance of marine and
terrestrial
fungi, caused by the sharp increase in the amount of dead plants and animals fed upon by the fungi.
[27]
For a while this "fungal spike" was used by some paleontologists to
identify the Permian–Triassic boundary in rocks that are unsuitable for
radiometric dating or lack suitable
index fossils,
but even the proposers of the fungal spike hypothesis pointed out that
"fungal spikes" may have been a repeating phenomenon created by the
post-extinction ecosystem in the earliest Triassic.
[27] The very idea of a fungal spike has been criticized on several grounds, including:
Reduviasporonites, the most common supposed fungal spore, was actually a fossilized
alga;
[18][28] the spike did not appear worldwide;
[29][30] and in many places it did not fall on the Permian–Triassic boundary.
[31]
The algae, which were misidentified as fungal spores, may even
represent a transition to a lake-dominated Triassic world rather than an
earliest Triassic zone of death and decay in some terrestrial fossil
beds.
[32] Newer chemical evidence agrees better with a fungal origin for
Reduviasporonites, diluting these critiques.
[33]
Uncertainty exists regarding the duration of the overall extinction
and about the timing and duration of various groups' extinctions within
the greater process. Some evidence suggests that there were multiple
extinction pulses
[7] or that the extinction was spread out over a few million years, with a sharp peak in the last million years of the Permian.
[31][34] Statistical analyses of some highly fossiliferous strata in Meishan,
Zhejiang Province in South China, suggest that the main extinction was clustered around one peak.
[11]
Recent research shows that different groups became extinct at different
times; for example, while difficult to date absolutely,
ostracod and
brachiopod extinctions were separated by 670 to 1170 thousand years.
[35] In a well-preserved sequence in east Greenland, the decline of animals is concentrated in a period 10 to
60 thousand years long, with plants taking several hundred thousand additional years to show the full impact of the event.
[36] An older theory, still supported in some recent papers,
[7][37]
is that there were two major extinction pulses 9.4 million years apart,
separated by a period of extinctions well above the background level,
and that the final extinction killed off only about 80% of marine
species alive at that time while the other losses occurred during the
first pulse or the interval between pulses. According to this theory one
of these extinction pulses occurred at the end of the
Guadalupian epoch of the Permian.
[7][38] For example, all but one of the surviving
dinocephalian genera died out at the end of the Guadalupian,
[39] as did the
Verbeekinidae, a family of large-size
fusuline foraminifera.
[40]
The impact of the end-Guadalupian extinction on marine organisms
appears to have varied between locations and between taxonomic
groups—brachiopods and corals had severe losses.
[41][42]
Extinction patterns
Marine organisms
Marine invertebrates
suffered the greatest losses during the P–Tr extinction. Evidence of
this was found in samples from south China sections at the P–Tr
boundary. Here, 286 out of 329 marine invertebrate genera disappear
within the final 2 sedimentary zones containing
conodonts from the Permian.
[11] The decrease in diversity was probably caused by a sharp increase in extinctions, rather than a decrease in
speciation.
[44]
The extinction primarily affected organisms with calcium carbonate skeletons, especially those reliant on stable CO
2 levels to produce their skeletons.
[45] These organisms were susceptible to the effects of the
ocean acidification that resulted from increased atmospheric CO
2.
Among
benthic
organisms, the extinction event multiplied background extinction rates,
and therefore caused most damage to taxa that had a high background
extinction rate (by implication, taxa with a high turnover).
[46][47] The extinction rate of marine organisms was catastrophic.
[11][48][49][50]
Surviving marine invertebrate groups include: articulate
brachiopods (those with a hinge),
[51] which have suffered a slow decline in numbers since the P–Tr extinction; the
Ceratitida order of
ammonites;
[52] and
crinoids ("sea lilies"),
[52] which very nearly became extinct but later became abundant and diverse.
The groups with the highest survival rates generally had active
control of circulation, elaborate gas exchange mechanisms, and light
calcification; more heavily calcified organisms with simpler breathing
apparatus were the worst hit.
[16][53] In the case of the brachiopods at least, surviving taxa were generally small, rare members of a diverse community.
[54]
The
ammonoids,
which had been in a long-term decline for the 30 million years since
the Roadian (middle Permian), suffered a selective extinction pulse 10
million years before the main event, at the end of the
Capitanian
stage. In this preliminary extinction, which greatly reduced disparity,
that is the range of different ecological guilds, environmental factors
were apparently responsible. Diversity and disparity fell further until
the P–Tr boundary; the extinction here was non-selective, consistent
with a catastrophic initiator. During the Triassic, diversity rose
rapidly, but disparity remained low.
[55]
The range of
morphospace
occupied by the ammonoids, that is the range of possible forms, shape
or structure, became more restricted as the Permian progressed. Just a
few million years into the Triassic, the original range of ammonoid
structures was once again reoccupied, but the parameters were now shared
differently among
clades.
[56]
Terrestrial invertebrates
The Permian had great diversity in insect and other invertebrate
species, including the largest insects ever to have existed. The
end-Permian is the only known mass extinction of insects,
[8] with eight or nine insect orders becoming extinct and ten more greatly reduced in diversity.
Palaeodictyopteroids
(insects with piercing and sucking mouthparts) began to decline during
the mid-Permian; these extinctions have been linked to a change in
flora. The greatest decline occurred in the Late Permian and was
probably not directly caused by weather-related floral transitions.
[48]
Most fossil insect groups found after the Permian–Triassic boundary
differ significantly from those that lived prior to the P–Tr extinction.
With the exception of the
Glosselytrodea,
Miomoptera, and
Protorthoptera, Paleozoic insect groups have not been discovered in deposits dating to after the P–Tr boundary. The
caloneurodeans,
monurans, paleodictyopteroids,
protelytropterans, and
protodonates
became extinct by the end of the Permian. In well-documented Late
Triassic deposits, fossils overwhelmingly consist of modern fossil
insect groups.
[8]
Terrestrial plants
Plant ecosystem response
The geological record of terrestrial plants is sparse and based mostly on
pollen and
spore
studies. Interestingly, plants are relatively immune to mass
extinction, with the impact of all the major mass extinctions
"insignificant" at a family level.
[18] Even the reduction observed in species diversity (of 50%) may be mostly due to
taphonomic processes.
[18]
However, a massive rearrangement of ecosystems does occur, with plant
abundances and distributions changing profoundly and all the forests
virtually disappearing;
[18][57] the Palaeozoic flora scarcely survived this extinction.
[58]
At the P–Tr boundary, the dominant floral groups changed, with many groups of land plants entering abrupt decline, such as
Cordaites (
gymnosperms) and
Glossopteris (
seed ferns).
[59] Dominant
gymnosperm genera were replaced post-boundary by
lycophytes—extant lycophytes are recolonizers of disturbed areas.
[60]
Palynological or pollen studies from East
Greenland of sedimentary rock strata laid down during the extinction period indicate dense gymnosperm
woodlands
before the event. At the same time that marine invertebrate macrofauna
declined, these large woodlands died out and were followed by a rise in
diversity of smaller
herbaceous plants including
Lycopodiophyta, both
Selaginellales and
Isoetales.
Later, other groups of gymnosperms again become dominant but again
suffered major die offs. These cyclical flora shifts occurred a few
times over the course of the extinction period and afterwards. These
fluctuations of the dominant flora between woody and herbaceous taxa
indicate chronic environmental stress resulting in a loss of most large
woodland plant species. The successions and extinctions of plant
communities do not coincide with the shift in
δ13C values, but occurred many years after.
[30] The recovery of gymnosperm forests took 4–5 million years.
[18]
Coal gap
No
coal deposits are known from the Early Triassic, and those in the Middle Triassic are thin and low-grade.
[19]
This "coal gap" has been explained in many ways. It has been suggested
that new, more aggressive fungi, insects and vertebrates evolved, and
killed vast numbers of trees. These decomposers themselves suffered
heavy losses of species during the extinction, and are not considered a
likely cause of the coal gap.
[19]
It could simply be that all coal forming plants were rendered extinct
by the P–Tr extinction, and that it took 10 million years for a new
suite of plants to adapt to the moist, acid conditions of
peat bogs.
[19] On the other hand,
abiotic factors (not caused by organisms), such as decreased rainfall or increased input of
clastic sediments, may also be to blame.
[18]
Finally, it is also true that there are very few sediments of any type
known from the Early Triassic, and the lack of coal may simply reflect
this scarcity. This opens the possibility that coal-producing
ecosystems
may have responded to the changed conditions by relocating, perhaps to
areas where we have no sedimentary record for the Early Triassic.
[18] For example, in eastern Australia a cold climate had been the norm for a long period of time, with a peat
mire ecosystem adapted to these conditions. Approximately 95% of these peat-producing plants went
locally extinct at the P–Tr boundary;
[61] Interestingly, coal deposits in Australia and Antarctica disappear significantly
before the P–Tr boundary.
[18]
Terrestrial vertebrates
There is enough evidence to indicate that over two-thirds of terrestrial
labyrinthodont amphibians,
sauropsid ("reptile") and
therapsid ("mammal-like reptile")
families became extinct. Large herbivores suffered the heaviest losses. All Permian
anapsid reptiles died out except the
procolophonids (
testudines have anapsid skulls but are most often thought to have evolved later, from diapsid ancestors).
Pelycosaurs
died out before the end of the Permian. Too few Permian diapsid fossils
have been found to support any conclusion about the effect of the
Permian extinction on
diapsids (the "reptile" group from which lizards, snakes, crocodilians, and
dinosaurs [including birds] evolved).
[62][63]
Even the groups that survived suffered extremely heavy losses of
species, and some terrestrial vertebrate groups very nearly became
extinct at the end-Permian. Some of the surviving groups did not persist
for long past this period, while others that barely survived went on to
produce diverse and long-lasting lineages. Yet it took 30
million years for the terrestrial vertebrate fauna to fully recover both numerically and ecologically.
[64]
Possible explanations of these patterns
An analysis of marine fossils from the Permian's final
Changhsingian stage found that marine organisms with low tolerance for
hypercapnia (high concentration of
carbon dioxide) had high extinction rates, while the most tolerant organisms had very slight losses.
The most vulnerable marine organisms were those that produced calcareous hard parts (i.e., from
calcium carbonate) and had low
metabolic rates and weak respiratory systems—notably
calcareous sponges,
rugose and
tabulate corals, calcite-depositing
brachiopods,
bryozoans, and
echinoderms; about 81% of such
genera became extinct. Close relatives without calcareous hard parts suffered only minor losses, for example
sea anemones,
from which modern corals evolved. Animals with high metabolic rates,
well-developed respiratory systems, and non-calcareous hard parts had
negligible losses—except for
conodonts, in which 33% of genera died out.
[65]
This pattern is consistent with what is known about the effects of
hypoxia, a shortage but not a total absence of
oxygen. However, hypoxia cannot have been the only killing mechanism for marine organisms. Nearly all of the
continental shelf
waters would have had to become severely hypoxic to account for the
magnitude of the extinction, but such a catastrophe would make it
difficult to explain the very selective pattern of the extinction.
Models
of the Late Permian and Early Triassic atmospheres show a significant
but protracted decline in atmospheric oxygen levels, with no
acceleration near the P–Tr boundary. Minimum atmospheric oxygen levels
in the Early Triassic are never less than present day levels—the decline
in oxygen levels does not match the temporal pattern of the extinction.
[65]
Marine organisms are more sensitive to changes in CO
2 levels than are terrestrial organisms for a variety of reasons. CO
2 is 28 times more soluble in water than is oxygen. Marine animals normally function with lower concentrations of CO
2 in their bodies than land animals, as the removal of CO
2
in air-breathing animals is impeded by the need for the gas to pass
through the respiratory system's membranes (lungs' alveolus,
tracheae, and the like), even when CO
2 diffuses more easily than oxygen. In marine organisms, relatively modest but sustained increases in CO
2 concentrations hamper the synthesis of
proteins, reduce fertilization rates, and produce deformities in calcareous hard parts.
[65] In addition, an increase in CO
2 concentration is inevitably linked to
ocean acidification,
consistent with the preferential extinction of heavily calcified taxa
and other signals in the rock record that suggest a more acidic ocean.
[66]
It is difficult to analyze extinction and survival rates of land
organisms in detail, because few terrestrial fossil beds span the
Permian–Triassic boundary. Triassic insects are very different from
those of the Permian, but a gap in the insect fossil record spans
approximately 15 million years from the late Permian to early Triassic.
The best-known record of vertebrate changes across the Permian–Triassic
boundary occurs in the
Karoo Supergroup of South Africa, but statistical analyses have so far not produced clear conclusions.
[65] However, analysis of the fossil river deposits of the floodplains indicate a shift from
meandering to
braided river patterns, indicating an abrupt drying of the climate.
[67] The climate change may have taken as little as 100,000 years, prompting the extinction of the unique
Glossopteris flora and its herbivores, followed by the carnivorous
guild.
[68] End-Permian extinctions did not occur at an instantaneous time horizon; particularly, floral extinction was delayed in time
[69]
Biotic recovery
Earlier analyses indicated that life on Earth recovered quickly after
the Permian extinctions, but this was mostly in the form of
disaster taxa, opportunist organisms such as the hardy
Lystrosaurus.
Research published in 2006 indicates that the specialized animals that
formed complex ecosystems, with high biodiversity, complex food webs and
a variety of niches, took much longer to recover. It is thought that
this long recovery was due to the successive waves of extinction, which
inhibited recovery, and prolonged environmental stress to organisms,
which continued into the Early Triassic. Research indicates that
recovery did not begin until the start of the mid-Triassic, 4 to 6
million years after the extinction;
[70] and some writers estimate that the recovery was not complete until
30 Ma after the P–Tr extinction, i.e. in the
late Triassic.
[7]
A study published in the journal
Science[71]
found that during the Great Extinction the oceans' surface temperatures
reached 40 °C (104 °F), which explains why recovery took so long: it
was simply too hot for life to survive.
[72] Of course, not all of the Earth's surface was 40°, and if it was simply too hot for life to survive then nothing
would have survived. Perhaps anoxia provides another aspect of what delayed the recovery.
[73]
During the early Triassic (4 to 6 million years after the P–Tr extinction), the plant biomass was insufficient to form
coal deposits, which implies a limited food mass for herbivores.
[19] River patterns in the
Karoo changed from
meandering to
braided, indicating that vegetation there was very sparse for a long time.
[74]
Each major segment of the early Triassic ecosystem—plant and animal, marine and terrestrial—was dominated by a small number of
genera, which appeared virtually worldwide, for example: the herbivorous
therapsid Lystrosaurus (which accounted for about 90% of early Triassic land vertebrates) and the
bivalves Claraia,
Eumorphotis,
Unionites and
Promylina. A healthy
ecosystem has a much larger number of genera, each living in a few preferred types of habitat.
[59][75]
Disaster taxa took advantage of the devastated ecosystems and enjoyed
a temporary population boom and increase in their territory.
Microconchids are the dominant component of otherwise impoverished Early Triassic encrusting assemblages. For example:
Lingula (a
brachiopod);
stromatolites, which had been confined to marginal environments since the
Ordovician;
Pleuromeia (a small, weedy plant);
Dicroidium (a
seed fern).
[75][76][77]
Changes in marine ecosystems
Sessile filter feeders like this
crinoid were significantly less abundant after the P–Tr extinction.
Prior to the extinction, about two-thirds of marine animals were
sessile
and attached to the sea floor but, during the Mesozoic, only about half
of the marine animals were sessile while the rest were free-living.
Analysis of marine fossils from the period indicated a decrease in the
abundance of sessile
epifaunal suspension feeders such as
brachiopods and
sea lilies and an increase in more complex mobile species such as
snails,
sea urchins and
crabs.
[78]
Before the Permian mass extinction event, both complex and simple
marine ecosystems were equally common; after the recovery from the mass
extinction, the complex communities outnumbered the simple communities
by nearly three to one,
[78] and the increase in predation pressure led to the
Mesozoic Marine Revolution.
Bivalves were fairly rare before the P–Tr extinction but became numerous and diverse in the Triassic, and one group, the
rudist clams, became the
Mesozoic's
main reef-builders. Some researchers think much of this change happened
in the 5 million years between the two major extinction pulses.
[79]
Crinoids ("sea lilies") suffered a selective extinction, resulting in a decrease in the variety of their forms. Their ensuing
adaptive radiation was brisk, and resulted in forms possessing flexible arms becoming widespread;
motility, predominantly a response to predation pressure, also became far more prevalent.
[81]
Land vertebrates
Lystrosaurus was by far the most abundant early Triassic land vertebrate.
Lystrosaurus, a pig-sized herbivorous
dicynodont therapsid, constituted as much as 90% of some earliest Triassic land vertebrate fauna. Smaller carnivorous
cynodont therapsids also survived, including the ancestors of
mammals. In the
Karoo region of southern
Africa, the
therocephalians Tetracynodon,
Moschorhinus and
Ictidosuchoides survived, but do not appear to have been abundant in the Triassic.
[82]
Archosaurs (which included the ancestors of dinosaurs and
crocodilians)
were initially rarer than therapsids, but they began to displace
therapsids in the mid-Triassic. In the mid to late Triassic, the
dinosaurs evolved from one group of archosaurs, and went on to dominate terrestrial ecosystems during the
Jurassic and
Cretaceous.
[83] This "Triassic Takeover" may have contributed to the
evolution of mammals by forcing the surviving therapsids and their
mammaliform successors to live as small, mainly nocturnal
insectivores; nocturnal life probably forced at least the mammaliforms to develop fur and higher
metabolic rates,
[84] while losing part of the differential color-sensitive retinal receptors reptilians and birds preserved.
Some
temnospondyl amphibians made a relatively quick recovery, in spite of nearly becoming extinct.
Mastodonsaurus and
trematosaurians were the main aquatic and semiaquatic predators during most of the
Triassic, some preying on
tetrapods and others on fish.
[85]
Land vertebrates took an unusually long time to recover from the P–Tr extinction; Palaeontologist
Michael Benton estimated the recovery was not complete until
30 million years after the extinction, i.e. not until the Late Triassic, in which dinosaurs,
pterosaurs, crocodiles, archosaurs, amphibians, and mammaliforms were abundant and diverse.
[5]
Causes
Pinpointing the exact cause or causes of the Permian–Triassic
extinction event is difficult, mostly because the catastrophe occurred
over 250 million years ago, and much of the evidence that would have
pointed to the cause has been destroyed by now or is concealed deep
within the Earth under many layers of rock. The
sea floor is also completely recycled every 200 million years by the ongoing process of
plate tectonics and
seafloor spreading,
leaving no useful indications beneath the ocean. With the fairly
significant evidence that scientists have accumulated, several
mechanisms have been proposed for the extinction event, including both
catastrophic and gradual processes (similar to those theorized for the
Cretaceous–Paleogene extinction event). The former group includes one or more large
bolide impact events, increased
volcanism, and sudden release of methane from the sea floor, either due to dissociation of
methane hydrate deposits or metabolism of organic carbon deposits by
methanogenic microbes. The latter group includes sea level change, increasing
anoxia, and increasing
aridity. Any hypothesis about the cause must explain the selectivity of the event, which affected organisms with
calcium carbonate
skeletons most severely; the long period (4 to 6 million years) before
recovery started, and the minimal extent of biological mineralization
(despite inorganic carbonates being deposited) once the recovery began.
[45]
Impact event
Artist's impression of a major impact event: A collision between Earth and an
asteroid a few kilometres in diameter would release as much energy as several million nuclear weapons detonating.
Evidence that an
impact event may have caused the
Cretaceous–Paleogene extinction event
(Cretaceous-Tertiary) has led to speculation that similar impacts may
have been the cause of other extinction events, including the P–Tr
extinction, and thus to a search for evidence of impacts at the times of
other extinctions and for large
impact craters of the appropriate age.
Reported evidence for an impact event from the P–Tr boundary level includes rare grains of
shocked quartz in Australia and Antarctica;
[86][87] fullerenes trapping extraterrestrial noble gases;
[88] meteorite fragments in Antarctica;
[89] and grains rich in iron, nickel and silicon, which may have been created by an impact.
[90] However, the accuracy of most of these claims has been challenged.
[91][92][93][94] Quartz from
Graphite Peak
in Antarctica, for example, once considered "shocked", has been
re-examined by optical and transmission electron microscopy. The
observed features were concluded to be not due to shock, but rather to
plastic deformation, consistent with formation in a
tectonic environment such as volcanism.
[95]
An impact crater on the sea floor would be evidence of a possible
cause of the P–Tr extinction, but such a crater would by now have
disappeared. As 70% of the Earth's surface is currently sea, an asteroid
or comet fragment is now perhaps more than twice as likely to hit ocean
as it is to hit land. However, Earth has no ocean-floor crust more than
200 million years old because the "conveyor belt" process of seafloor
spreading and
subduction destroys it within that time. Craters produced by very large impacts may be masked by extensive
flood basalting from below after the crust is punctured or weakened.
[96]
Subduction should not, however, be entirely accepted as an explanation
of why no firm evidence can be found: as with the K-T event, an ejecta
blanket stratum rich in
siderophilic elements (such as
iridium) would be expected to be seen in formations from the time.
One attraction of large impact theories is that theoretically they
could trigger other cause-considered extinction-paralleling phenomena,
[clarification needed][97] such as the
Siberian Traps eruptions (see below) as being either an impact site
[98] or the
antipode of an impact site.
[97][99] The abruptness of an impact also explains why more species did not
rapidly evolve to survive, as would be expected if the Permian-Triassic event had been slower and less global than a meteorite impact.
Possible impact sites
Several possible impact craters have been proposed as the site of an impact causing the P–Tr extinction, including the
Bedout structure off the northwest coast of Australia
[87] and the hypothesized
Wilkes Land crater of East Antarctica.
[100][101]
In each case, the idea that an impact was responsible has not been
proven and has been widely criticized. In the case of Wilkes Land, the
age of this sub-ice geophysical feature is very uncertain – it may be
later than the Permian–Triassic extinction.
The
Araguainha crater
in Brazil has been most recently dated to 254.7 ± 2.5 million years
ago, overlapping with estimates for the Permo-Triassic boundary.
[102] Much of the local rock was
oil shale.
The estimated energy released by the Araguainha impact is insufficient
to be a direct cause of the global mass extinction, but the colossal
local earth tremors would have released huge amounts of oil and gas from
the shattered rock. The resulting
sudden global warming might have precipitated the Permian–Triassic extinction event.
[103]
Volcanism
The final stages of the Permian had two
flood basalt events. A small one, the
Emeishan Traps in
China, occurred at the same time as the end-
Guadalupian extinction pulse, in an area close to the equator at the time.
[104][105] The flood basalt eruptions that produced the
Siberian Traps
constituted one of the largest known volcanic events on Earth and
covered over 2,000,000 square kilometres (770,000 sq mi) with lava.
[106][107][108] The date of the Siberian Traps eruptions and the extinction event are in good agreement.
[21][109]
The Emeishan and Siberian Traps eruptions may have caused dust clouds and acid
aerosols, which would have blocked out sunlight and thus disrupted photosynthesis both on land and in the
photic zone
of the ocean, causing food chains to collapse. The eruptions may also
have caused acid rain when the aerosols washed out of the atmosphere.
That may have killed land plants and
molluscs and
planktonic organisms which had
calcium carbonate shells. The eruptions would also have emitted
carbon dioxide, causing
global warming.
When all of the dust clouds and aerosols washed out of the atmosphere,
the excess carbon dioxide would have remained and the warming would have
proceeded without any mitigating effects.
[97]
The Siberian Traps had unusual features that made them even more
dangerous. Pure flood basalts produce fluid, low-viscosity lava and do
not hurl debris into the atmosphere. It appears, however, that 20% of
the output of the Siberian Traps eruptions was
pyroclastic (consisted of ash and other debris thrown high into the atmosphere), increasing the short-term cooling effect.
[110] The basalt lava erupted or intruded into
carbonate
rocks and into sediments that were in the process of forming large coal
beds, both of which would have emitted large amounts of carbon dioxide,
leading to stronger global warming after the dust and aerosols settled.
[97]
In January 2011, a team, led by Stephen Grasby of the Geological
Survey of Canada—Calgary, reported evidence that volcanism caused
massive coal beds to ignite, possibly releasing more than 3 trillion
tons of carbon. The team found ash deposits in deep rock layers near
what is now
Buchanan Lake.
According to their article, "coal ash dispersed by the explosive
Siberian Trap eruption would be expected to have an associated release
of toxic elements in impacted water bodies where fly ash slurries
developed.... Mafic megascale eruptions are long-lived events that would
allow significant build-up of global ash clouds."
[111][112]
In a statement, Grasby said, "In addition to these volcanoes causing
fires through coal, the ash it spewed was highly toxic and was released
in the land and water, potentially contributing to the worst extinction
event in earth history."
[113] In 2013, QY Tang reported the total amounts of important volatiles emitted from the Siberian Traps are 8.5 × 10
7 Tg CO2, 4.4 × 10
6 Tg CO, 7.0 × 10
6 Tg H2S and 6.8 × 10
7
Tg SO2, the data support a popular notion that the end-Permian mass
extinction on the Earth was caused by the emission of enormous amounts
of volatiles from the Siberian Traps into the atmosphere.
[114]
In 2015, evidence and a timeline indicated the extinction was caused by events in the
Large Igneous Province of the Siberian Traps.
[115][116][117][118][119]
Methane hydrate gasification
Scientists have found worldwide evidence of a swift decrease of about 1% in the
13C/
12C isotope ratio in
carbonate rocks from the end-Permian.
[50][120] This is the first, largest, and most rapid of a series of negative and positive excursions (decreases and increases in
13C/
12C
ratio) that continues until the isotope ratio abruptly stabilised in
the middle Triassic, followed soon afterwards by the recovery of
calcifying life forms (organisms that use
calcium carbonate to build hard parts such as shells).
[16]
A variety of factors may have contributed to this drop in the
13C/
12C ratio, but most turn out to be insufficient to account fully for the observed amount:
[121]
- Gases from volcanic eruptions have a 13C/12C ratio about 0.5 to 0.8% below standard (δ13C
about −0.5 to −0.8%), but an assessment made in 1995 concluded that the
amount required to produce a reduction of about 1.0% worldwide requires
eruptions greater by orders of magnitude than any for which evidence has been found.[122] (However, this analysis addressed only CO2 produced by the magma itself, not from interactions with carbon bearing sediments, as later proposed.)
- A reduction in organic activity would extract 12C more slowly from the environment and leave more of it to be incorporated into sediments, thus reducing the 13C/12C
ratio. Biochemical processes preferentially use the lighter isotopes
since chemical reactions are ultimately driven by electromagnetic forces
between atoms and lighter isotopes respond more quickly to these
forces, but a study of a smaller drop of 0.3 to 0.4% in 13C/12C (δ13C −3 to −4 ‰) at the Paleocene-Eocene Thermal Maximum
(PETM) concluded that even transferring all the organic carbon (in
organisms, soils, and dissolved in the ocean) into sediments would be
insufficient: even such a large burial of material rich in 12C would not have produced the 'smaller' drop in the 13C/12C ratio of the rocks around the PETM.[122]
- Buried sedimentary organic matter has a 13C/12C ratio 2.0 to 2.5% below normal (δ13C
−2.0 to −2.5%). Theoretically, if the sea level fell sharply, shallow
marine sediments would be exposed to oxidization. But 6500–8400 gigatons (1 gigaton = 109 metric tons)
of organic carbon would have to be oxidized and returned to the
ocean-atmosphere system within less than a few hundred thousand years to
reduce the 13C/12C ratio by 1.0%, which is not thought to be a realistic possibility.[48] Moreover, sea levels were rising rather than falling at the time of the extinction.[123]
- Rather than a sudden decline in sea level, intermittent periods of ocean-bottom hyperoxia and anoxia (high-oxygen and low- or zero-oxygen conditions) may have caused the 13C/12C ratio fluctuations in the Early Triassic;[16]
and global anoxia may have been responsible for the end-Permian blip.
The continents of the end-Permian and early Triassic were more clustered
in the tropics than they are now, and large tropical rivers would have
dumped sediment into smaller, partially enclosed ocean basins at low
latitudes. Such conditions favor oxic and anoxic episodes; oxic/anoxic
conditions would result in a rapid release/burial, respectively, of
large amounts of organic carbon, which has a low 13C/12C ratio because biochemical processes use the lighter isotopes more.[124]
That or another organic-based reason may have been responsible for both
that and a late Proterozoic/Cambrian pattern of fluctuating 13C/12C ratios.[16]
Other hypotheses include mass oceanic poisoning releasing vast amounts of CO
2[125] and a long-term reorganisation of the global carbon cycle.
[121]
Prior to consideration of the inclusion of roasting carbonate
sediments by volcanism, the only proposed mechanism sufficient to cause a
global 1% reduction in the
13C/
12C ratio was the release of
methane from
methane clathrates,.
[48] Carbon-cycle models confirm that it would have had enough effect to produce the observed reduction.
[121][125]
Methane clathrates, also known as methane hydrates, consist of methane
molecules trapped in cages of water molecules. The methane, produced by
methanogens (microscopic single-celled organisms), has a
13C/
12C ratio about 6.0% below normal (
δ13C −6.0%). At the right combination of pressure and temperature, it gets trapped in clathrates fairly close to the surface of
permafrost and in much larger quantities at continental margins (
continental shelves
and the deeper seabed close to them). Oceanic methane hydrates are
usually found buried in sediments where the seawater is at least 300 m
(980 ft) deep. They can be found up to about 2,000 m (6,600 ft) below
the sea floor, but usually only about 1,100 m (3,600 ft) below the sea
floor.
[126]
The area covered by lava from the Siberian Traps eruptions is about
twice as large as was originally thought, and most of the additional
area was shallow sea at the time. The seabed probably contained
methane hydrate deposits, and the lava caused the deposits to dissociate, releasing vast quantities of methane.
[127] A vast release of methane might cause significant global warming since methane is a very powerful
greenhouse gas.
Strong evidence suggests the global temperatures increased by about
6 °C (10.8 °F) near the equator and therefore by more at higher
latitudes: a sharp decrease in oxygen isotope ratios (
18O/
16O);
[128] the extinction of
Glossopteris flora (
Glossopteris
and plants that grew in the same areas), which needed a cold climate,
with its replacement by floras typical of lower paleolatitudes.
[129]
However, the pattern of isotope shifts expected to result from a
massive release of methane does not match the patterns seen throughout
the early Triassic. Not only would such a cause require the release of
five times as much methane as postulated for the PETM,
[16] but would it also have to be reburied at an unrealistically high rate to account for the rapid increases in the
13C/
12C ratio (episodes of high positive
δ13C) throughout the early Triassic before it was released again several times.
[16]
Anoxia
Evidence for widespread ocean
anoxia (severe deficiency of oxygen) and
euxinia (presence of
hydrogen sulfide) is found from the Late Permian to the Early Triassic. Throughout most of the
Tethys and
Panthalassic Oceans, evidence for anoxia, including fine laminations in sediments, small pyrite
framboids, high uranium/thorium ratios, and
biomarkers for
green sulfur bacteria, appear at the extinction event.
[130] However, in some sites, including
Meishan, China, and eastern Greenland, evidence for anoxia precedes the extinction.
[131][132] Biomarkers for green sulfur bacteria, such as isorenieratane, the
diagenetic product of
isorenieratene, are widely used as indicators of
photic zone
euxinia because green sulfur bacteria require both sunlight and
hydrogen sulfide to survive. Their abundance in sediments from the P-T
boundary indicates hydrogen sulfide was present even in shallow waters.
This spread of toxic, oxygen-depleted water would have been
devastating for marine life, producing widespread die-offs. Models of
ocean chemistry show that anoxia and euxinia would have been closely
associated with
hypercapnia (high levels of carbon dioxide).
[133]
This suggests that poisoning from hydrogen sulfide, anoxia, and
hypercapnia acted together as a killing mechanism. Hypercapnia best
explains the selectivity of the extinction, but anoxia and euxinia
probably contributed to the high mortality of the event. The persistence
of anoxia through the Early Triassic may explain the slow recovery of
marine life after the extinction. Models also show that anoxic events
can cause catastrophic hydrogen sulfide emissions into the atmosphere
(see below).
[134]
The sequence of events leading to anoxic oceans may have been triggered by carbon dioxide emissions from the eruption of the
Siberian Traps.
[134]
In that scenario, warming from the enhanced greenhouse effect would
reduce the solubility of oxygen in seawater, causing the concentration
of oxygen to decline. Increased weathering of the continents due to
warming and the acceleration of the
water cycle
would increase the riverine flux of phosphate to the ocean. The
phosphate would have supported greater primary productivity in the
surface oceans. The increase in organic matter production would have
caused more organic matter to sink into the deep ocean, where its
respiration would further decrease oxygen concentrations. Once anoxia
became established, it would have been sustained by a
positive feedback loop because deep water anoxia tends to increase the recycling efficiency of phosphate, leading to even higher productivity.
Hydrogen sulfide emissions
A severe
anoxic event at the end of the Permian would have allowed
sulfate-reducing bacteria
to thrive, causing the production of large amounts of hydrogen sulfide
in the anoxic ocean. Upwelling of this water may have released massive
hydrogen sulfide emissions into the atmosphere and would poison terrestrial plants and animals and severely weaken the
ozone layer, exposing much of the life that remained to fatal levels of
UV radiation.
[134] Indeed,
biomarker evidence for anaerobic photosynthesis by
Chlorobiaceae
(green sulfur bacteria) from the Late-Permian into the Early Triassic
indicates that hydrogen sulfide did upwell into shallow waters because
these bacteria are restricted to the photic zone and use sulfide as an
electron donor.
The hypothesis has the advantage of explaining the mass extinction of
plants, which would have added to the methane levels and should
otherwise have thrived in an atmosphere with a high level of carbon
dioxide. Fossil spores from the end-Permian further support the theory:
[citation needed] many show deformities that could have been caused by
ultraviolet radiation, which would have been more intense after hydrogen sulfide emissions weakened the ozone layer.
The supercontinent Pangaea
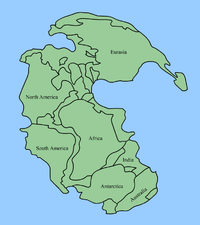
Map of
Pangaea showing where today's continents were at the Permian–Triassic boundary
About halfway through the Permian (in the
Kungurian age of the Permian's
Cisuralian epoch), all the continents joined to form the supercontinent
Pangaea, surrounded by the
superocean Panthalassa, although blocks that are now parts of Asia did not join the supercontinent until very late in the Permian.
[135]
The configuration severely decreased the extent of shallow aquatic
environments, the most productive part of the seas, and it exposed
formerly isolated organisms of the rich continental shelves to
competition from invaders. Pangaea's formation would also have altered
both oceanic circulation and atmospheric weather patterns, creating
seasonal
monsoons near the coasts and an arid climate in the vast continental interior.
[citation needed]
Marine life suffered very high but not catastrophic rates of
extinction after the formation of Pangaea (see the diagram "Marine genus
biodiversity" at the top of this article), almost as high as in some of
the
"Big Five" mass extinctions.
The formation of Pangaea seems not to have caused a significant rise in
extinction levels on land, and, in fact, most of the advance of the
therapsids
and increase in their diversity seems to have occurred in the late
Permian, after Pangaea was almost complete. Thus, it seems likely that
Pangaea initiated a long period of increased marine extinctions but was
not directly responsible for the "Great Dying" and the end of the
Permian.
Microbes
A hypothesis published in 2014 posits that a genus of
anaerobic methanogenic archaea known as
Methanosarcina was responsible for the event.
[136] Three lines of evidence suggest that these microbes acquired a new metabolic pathway via
gene transfer
at about that time, enabling them to efficiently metabolize acetate
into methane. That would have led to their exponential reproduction,
allowing them to rapidly consume vast deposits of organic carbon that
had accumulated in the marine sediment. The result would have been a
sharp buildup of methane and carbon dioxide in the Earth's oceans and
atmosphere, in a manner that may be consistent with the
13C/
12C
isotopic record. Massive volcanism facilitated this process by
releasing large amounts of nickel, a scarce metal which is a cofactor
for an enzymes involved in producing methane.
[136] On the other hand, in the canonical Meishan sections, the Nickel concentration increases somewhat after the
δ13C concentrations have begun to fall.
[137]
Combination of causes
Possible causes supported by strong evidence appear to describe a
sequence of catastrophes, each worse than the last: the Siberian Traps
eruptions were bad enough alone, but because they occurred near coal
beds and the continental shelf, they also triggered very large releases
of carbon dioxide and methane.
[65]
The resultant global warming may have caused perhaps the most severe
anoxic event in the oceans' history: according to this theory, the
oceans became so anoxic, anaerobic sulfur-reducing organisms dominated
the chemistry of the oceans and caused massive emissions of toxic
hydrogen sulfide.
[65]
However, there may be some weak links in this chain of events: the changes in the
13C/
12C ratio expected to result from a massive release of methane do not match the patterns seen throughout the early Triassic;
[16] and the types of oceanic
thermohaline circulation that may have existed at the end of the Permian are not likely to have supported deep-sea anoxia.
[138]
See also
References
Rohde, R.A. & Muller, R.A. (2005). "Cycles in fossil diversity". Nature 434 (7030): 209–210. Bibcode:2005Natur.434..208R. doi:10.1038/nature03339. PMID 15758998.
Further reading
- Over, Jess (editor), Understanding Late Devonian and Permian–Triassic Biotic and Climatic Events,
(Volume 20 in series Developments in Palaeontology and Stratigraphy
(2006). The state of the inquiry into the extinction events.
- Sweet, Walter C. (editor), Permo–Triassic Events in the Eastern Tethys : Stratigraphy Classification and Relations with the Western Tethys (in series World and Regional Geology)
External links
""Great Dying" Lasted 200,000 Years". National Geographic. 23 November 2011. Retrieved 1 April 2014.
"How a Single Act of Evolution Nearly Wiped Out All Life on Earth". ScienceDaily. 1 April 2014. Retrieved 1 April 2014.
Shen S.-Z.; et al. (2011). "Calibrating the End-Permian Mass Extinction". Science 334 (6061): 1367–1372. Bibcode:2011Sci...334.1367S. doi:10.1126/science.1213454. PMID 22096103.
Benton M J (2005). When life nearly died: the greatest mass extinction of all time. London: Thames & Hudson. ISBN 0-500-28573-X.
Carl T. Bergstrom; Lee Alan Dugatkin (2012). Evolution. Norton. p. 515. ISBN 978-0-393-92592-0.
Sahney, S.; Benton, M. J. (2008). "Recovery from the most profound mass extinction of all time". Proceedings of the Royal Society: Biological 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
Labandeira CC, Sepkoski JJ (1993). "Insect diversity in the fossil record". Science 261 (5119): 310–315. Bibcode:1993Sci...261..310L. doi:10.1126/science.11536548. PMID 11536548.
Sole RV, Newman M (2003). "Extinctions and Biodiversity in the Fossil Record". In Canadell JG, Mooney, HA. Encyclopedia
of Global Environmental Change, The Earth System – Biological and
Ecological Dimensions of Global Environmental Change (Volume 2). New York: Wiley. pp. 297–391. ISBN 0-470-85361-1.
"It Took Earth Ten Million Years to Recover from Greatest Mass Extinction". ScienceDaily. 27 May 2012. Retrieved 28 May 2012.
Jin
YG, Wang Y, Wang W, Shang QH, Cao CQ, Erwin DH (2000). "Pattern of
Marine Mass Extinction Near the Permian–Triassic Boundary in South
China". Science 289 (5478): 432–436. Bibcode:2000Sci...289..432J. doi:10.1126/science.289.5478.432. PMID 10903200.
Yin H, Zhang K, Tong J, Yang Z, Wu S. The Global Stratotype Section and Point (GSSP) of the Permian-Triassic Boundary. Episodes 24. pp. 102–114.
Yin HF, Sweets WC, Yang ZY, Dickins JM (1992). "Permo-Triassic events in the eastern Tethys–an overview". In Sweet WC. Permo-Triassic events in the eastern Tethys: stratigraphy, classification, and relations with the western Tethys. Cambridge, UK: Cambridge University Press. pp. 1–7. ISBN 0-521-54573-0.
Darcy E. Ogdena and Norman H. Sleep (2011). "Explosive eruption of coal and basalt and the end-Permian mass extinction". Proceedings of the National Academy of Sciences of the United States of America 109 (1): 59–62. Bibcode:2012PNAS..109...59O. doi:10.1073/pnas.1118675109. PMC 3252959. PMID 22184229.
David L. Chandler – MIT News Office (31 March 2014). "Ancient whodunit may be solved: The microbes did it!". MIT News.
Payne, J. L.; Lehrmann, D. J.; Wei, J.; Orchard, M. J.; Schrag, D. P.; Knoll, A. H. (2004). "Large Perturbations of the Carbon Cycle During Recovery from the End-Permian Extinction". Science 305 (5683): 506–9. doi:10.1126/science.1097023. PMID 15273391.
Benton, M. J. (2012). "No gap in the Middle Permian record of terrestrial vertebrates". Geology 40. doi:10.1130/g32669.1.
McElwain, J. C.; Punyasena, S. W. (2007). "Mass extinction events and the plant fossil record". Trends in Ecology & Evolution 22 (10): 548–557. doi:10.1016/j.tree.2007.09.003. PMID 17919771.
Retallack,
G. J.; Veevers, J. J.; Morante, R. (1996). "Global coal gap between
Permian–Triassic extinctions and middle Triassic recovery of peat
forming plants". GSA Bulletin 108 (2): 195–207. doi:10.1130/0016-7606(1996)108<0195:gcgbpt>2.3.CO;2.
Erwin, D.H (1993). The Great Paleozoic Crisis: Life and Death in the Permian. New York: Columbia University Press. ISBN 0-231-07467-0.
Burgess, SD (2014). "High-precision timeline for Earth's most severe extinction". Nature 111 (9): 3316–3321. doi:10.1073/pnas.1317692111. PMC 3948271. PMID 24516148.
Magaritz M (1989). "13C minima follow extinction events: a clue to faunal radiation". Geology 17 (4): 337–340. Bibcode:1989Geo....17..337M. doi:10.1130/0091-7613(1989)017<0337:cmfeea>2.3.CO;2.
Krull SJ, Retallack JR (2000). "13C depth profiles from paleosols across the Permian–Triassic boundary: Evidence for methane release". GSA Bulletin 112 (9): 1459–1472. Bibcode:2000GSAB..112.1459K. doi:10.1130/0016-7606(2000)112<1459:cdpfpa>2.0.CO;2. ISSN 0016-7606.
Dolenec
T, Lojen S, Ramovs A (2001). "The Permian–Triassic boundary in Western
Slovenia (Idrijca Valley section): magnetostratigraphy, stable isotopes,
and elemental variations". Chemical Geology 175 (1): 175–190. doi:10.1016/S0009-2541(00)00368-5.
Musashi
M, Isozaki Y, Koike T, Kreulen R (2001). "Stable carbon isotope
signature in mid-Panthalassa shallow-water carbonates across the
Permo–Triassic boundary: evidence for 13C-depleted ocean". Earth Planet. Sci. Lett. 193: 9–20. Bibcode:2001E&PSL.191....9M. doi:10.1016/S0012-821X(01)00398-3.
Dolenec
T, Lojen S, Ramovs A (2001). "The Permian-Triassic boundary in Western
Slovenia (Idrijca Valley section): magnetostratigraphy, stable isotopes,
and elemental variations". Chemical Geology 175: 175–190. doi:10.1016/S0009-2541(00)00368-5.
H Visscher, H Brinkhuis, D L Dilcher, W C Elsik, Y Eshet, C V Looy, M R Rampino, and A Traverse (1996). "The terminal Paleozoic fungal event: Evidence of terrestrial ecosystem destabilization and collapse". Proceedings of the National Academy of Sciences 93 (5): 2155–2158. Bibcode:1996PNAS...93.2155V. doi:10.1073/pnas.93.5.2155. PMC 39926. PMID 11607638.
Foster, C.B.; Stephenson, M.H.; Marshall, C.; Logan, G.A.; Greenwood, P.F. (2002). "A Revision Of Reduviasporonites Wilson 1962: Description, Illustration, Comparison And Biological Affinities". Palynology 26 (1): 35–58. doi:10.2113/0260035.
López-Gómez, J. and Taylor, E.L. (2005). "Permian-Triassic Transition in Spain: A multidisciplinary approach". Palaeogeography, Palaeoclimatology, Palaeoecology 229 (1–2): 1–2. doi:10.1016/j.palaeo.2005.06.028.
Looy CV, Twitchett RJ, Dilcher DL, Van Konijnenburg-Van Cittert JH, Visscher H (2005). "Life in the end-Permian dead zone". Proceedings of the National Academy of Sciences 162 (4): 7879–7883. Bibcode:2001PNAS...98.7879L. doi:10.1073/pnas.131218098. PMC 35436. PMID 11427710. See image 2
Ward
PD, Botha J, Buick R, De Kock MO, Erwin DH, Garrison GH, Kirschvink JL
& Smith R (2005). "Abrupt and Gradual Extinction Among Late Permian
Land Vertebrates in the Karoo Basin, South Africa". Science 307 (5710): 709–714. Bibcode:2005Sci...307..709W. doi:10.1126/science.1107068. PMID 15661973.
Retallack, G.J.; Smith, R.M.H.; Ward, P.D. (2003). "Vertebrate extinction across Permian-Triassic boundary in Karoo Basin, South Africa". Bulletin of the Geological Society of America 115 (9): 1133–1152. Bibcode:2003GSAB..115.1133R. doi:10.1130/B25215.1.
Sephton, M. A.; Visscher, H.; Looy, C. V.; Verchovsky, A. B.; Watson, J. S. (2009). "Chemical constitution of a Permian-Triassic disaster species". Geology 37 (10): 875–878. doi:10.1130/G30096A.1.
Rampino
MR, Prokoph A & Adler A (2000). "Tempo of the end-Permian event:
High-resolution cyclostratigraphy at the Permian–Triassic boundary". Geology 28 (7): 643–646. Bibcode:2000Geo....28..643R. doi:10.1130/0091-7613(2000)28<643:toteeh>2.0.CO;2. ISSN 0091-7613.
Wang, S.C.; Everson, P.J. (2007). "Confidence intervals for pulsed mass extinction events". Paleobiology 33 (2): 324–336. doi:10.1666/06056.1.
Twitchett
RJ Looy CV Morante R Visscher H & Wignall PB (2001). "Rapid and
synchronous collapse of marine and terrestrial ecosystems during the
end-Permian biotic crisis". Geology 29 (4): 351–354. Bibcode:2001Geo....29..351T. doi:10.1130/0091-7613(2001)029<0351:rascom>2.0.CO;2. ISSN 0091-7613.
Retallack,
G.J.; Metzger, C.A.; Greaver, T.; Jahren, A.H.; Smith, R.M.H.; Sheldon,
N.D. (2006). "Middle-Late Permian mass extinction on land". Bulletin of the Geological Society of America 118 (11–12): 1398–1411. Bibcode:2006GSAB..118.1398R. doi:10.1130/B26011.1.
Stanley SM & Yang X (1994). "A Double Mass Extinction at the End of the Paleozoic Era". Science 266 (5189): 1340–1344. Bibcode:1994Sci...266.1340S. doi:10.1126/science.266.5189.1340. PMID 17772839.
Retallack,
G.J., Metzger, C.A., Jahren, A.H., Greaver, T., Smith, R.M.H., and
Sheldon, N.D (November–December 2006). "Middle-Late Permian mass
extinction on land". GSA Bulletin 118 (11/12): 1398–1411. Bibcode:2006GSAB..118.1398R. doi:10.1130/B26011.1.
Ota,
A, and Isozaki, Y. (March 2006). "Fusuline biotic turnover across the
Guadalupian–Lopingian (Middle–Upper Permian) boundary in mid-oceanic
carbonate buildups: Biostratigraphy of accreted limestone in Japan". Journal of Asian Earth Sciences 26 (3–4): 353–368. Bibcode:2006JAESc..26..353O. doi:10.1016/j.jseaes.2005.04.001.
Shen,
S., and Shi, G.R. (2002). "Paleobiogeographical extinction patterns of
Permian brachiopods in the Asian-western Pacific region". Paleobiology 28 (4): 449–463. doi:10.1666/0094-8373(2002)028<0449:pepopb>2.0.CO;2. ISSN 0094-8373.
Wang, X-D, and Sugiyama, T. (December 2000). "Diversity and extinction patterns of Permian coral faunas of China". Lethaia 33 (4): 285–294. doi:10.1080/002411600750053853.
Racki G (1999). "Silica-secreting biota and mass extinctions: survival processes and patterns". Palaeogeography, Palaeoclimatology, Palaeoecology 154 (1–2): 107–132. doi:10.1016/S0031-0182(99)00089-9.
Bambach, R.K.; Knoll, A.H.; Wang, S.C. (December 2004). "Origination, extinction, and mass depletions of marine diversity". Paleobiology 30 (4): 522–542. doi:10.1666/0094-8373(2004)030<0522:oeamdo>2.0.CO;2. ISSN 0094-8373.
Knoll, A.H. (2004). "Biomineralization
and evolutionary history. In: P.M. Dove, J.J. DeYoreo and S. Weiner
(Eds), Reviews in Mineralogy and Geochemistry," (PDF).
Stanley, S.M. (2008). "Predation defeats competition on the seafloor". Paleobiology 34 (1): 1–21. doi:10.1666/07026.1. Retrieved 2008-05-13.
Stanley, S.M. (2007). "An Analysis of the History of Marine Animal Diversity". Paleobiology 33 (sp6): 1–55. doi:10.1666/06020.1.
Erwin DH (1993). The great Paleozoic crisis; Life and death in the Permian. Columbia University Press. ISBN 0-231-07467-0.
McKinney, M.L. (1987). "Taxonomic selectivity and continuous variation in mass and background extinctions of marine taxa". Nature 325 (6100): 143–145. Bibcode:1987Natur.325..143M. doi:10.1038/325143a0.
Twitchett
RJ, Looy CV, Morante R, Visscher H, Wignall PB (2001). "Rapid and
synchronous collapse of marine and terrestrial ecosystems during the
end-Permian biotic crisis". Geology 29 (4): 351–354. Bibcode:2001Geo....29..351T. doi:10.1130/0091-7613(2001)029<0351:rascom>2.0.CO;2. ISSN 0091-7613.
"Permian : The Marine Realm and The End-Permian Extinction". paleobiology.si.edu. Retrieved 2016-01-26.
"Permian extinction". Encyclopedia Britannica. Retrieved 2016-01-26.
Knoll,
A.H.; Bambach, R.K.; Canfield, D.E.; Grotzinger, J.P. (1996).
"Comparative Earth history and Late Permian mass extinction". Science 273 (5274): 452–457. Bibcode:1996Sci...273..452K. doi:10.1126/science.273.5274.452. PMID 8662528.
Leighton,
L.R.; Schneider, C.L. (2008). "Taxon characteristics that promote
survivorship through the Permian–Triassic interval: transition from the
Paleozoic to the Mesozoic brachiopod fauna". Paleobiology 34 (1): 65–79. doi:10.1666/06082.1.
Villier, L.; Korn, D. (Oct 2004). "Morphological Disparity of Ammonoids and the Mark of Permian Mass Extinctions". Science 306 (5694): 264–266. Bibcode:2004Sci...306..264V. doi:10.1126/science.1102127. ISSN 0036-8075. PMID 15472073.
Saunders,
W. B.; Greenfest-Allen, E.; Work, D. M.; Nikolaeva, S. V. (2008).
"Morphologic and taxonomic history of Paleozoic ammonoids in time and
morphospace". Paleobiology 34 (1): 128–154. doi:10.1666/07053.1.
"The Dino Directory – Natural History Museum".
Cascales-Miñana, B.; Cleal, C. J. (2011). "Plant fossil record and survival analyses". Lethaia 45: no–no. doi:10.1111/j.1502-3931.2011.00262.x.
Retallack, GJ (1995). "Permian–Triassic life crisis on land". Science 267 (5194): 77–80. Bibcode:1995Sci...267...77R. doi:10.1126/science.267.5194.77. PMID 17840061.
Looy, CV Brugman WA Dilcher DL & Visscher H (1999). "The delayed resurgence of equatorial forests after the Permian–Triassic ecologic crisis". Proceedings of the National Academy of Sciences of the United States of America 96 (24): 13857–13862. Bibcode:1999PNAS...9613857L. doi:10.1073/pnas.96.24.13857. PMC 24155. PMID 10570163.
Michaelsen
P (2002). "Mass extinction of peat-forming plants and the effect on
fluvial styles across the Permian–Triassic boundary, northern Bowen
Basin, Australia". Palaeogeography, Palaeoclimatology, Palaeoecology 179 (3–4): 173–188. doi:10.1016/S0031-0182(01)00413-8.
Maxwell, W. D. (1992). "Permian and Early Triassic extinction of non-marine tetrapods". Palaeontology 35: 571–583.
Erwin DH (1990). "The End-Permian Mass Extinction". Annual Review of Ecology and Systematics 21: 69–91. doi:10.1146/annurev.es.21.110190.000441.
"Bristol University – News – 2008: Mass extinction".
Knoll, A.H., Bambach, R.K., Payne, J.L., Pruss, S., and Fischer, W.W. (2007). "Paleophysiology and end-Permian mass extinction" (PDF). Earth and Planetary Science Letters 256 (3–4): 295–313. Bibcode:2007E&PSL.256..295K. doi:10.1016/j.epsl.2007.02.018. Retrieved 2008-07-04.
Payne, J.; Turchyn, A.; Paytan, A.; Depaolo, D.; Lehrmann, D.; Yu, M.; Wei, J. (2010). "Calcium isotope constraints on the end-Permian mass extinction". Proceedings of the National Academy of Sciences of the United States of America 107 (19): 8543–8548. Bibcode:2010PNAS..107.8543P. doi:10.1073/pnas.0914065107. PMC 2889361. PMID 20421502.
Smith, R.M.H. (16 November 1999). "Changing
fluvial environments across the Permian-Triassic boundary in the Karoo
Basin, South Africa and possible causes of tetrapod extinctions". Palaeogeography, Palaeoclimatology, Palaeoecology 117 (1-2): 81–104. doi:10.1016/0031-0182(94)00119-S. Retrieved 21 February 2012.
Chinsamy-Turan (2012). Anusuya, ed. Forerunners of mammals : radiation, histology, biology. Bloomington: Indiana University Press. ISBN 978-0-253-35697-0.
Visscher,
Henk; Looy, Cindy V.; Collinson, Margaret E.; Brinkhuis, Henk; Cittert,
Johanna H. A. van Konijnenburg-van; Kürschner, Wolfram M.; Sephton,
Mark A. (2004-08-31). "Environmental mutagenesis during the end-Permian ecological crisis". Proceedings of the National Academy of Sciences of the United States of America 101 (35): 12952–12956. doi:10.1073/pnas.0404472101. ISSN 0027-8424. PMC 516500. PMID 15282373.
Lehrmann,
Daniel J.; Ramezani, Jahandar; Bowring, Samuel A.; Martin, Mark W.;
Montgomery, Paul; Enos, Paul; Payne, Jonathan L.; Orchard, Michael J.;
Wang Hongmei; Wei Jiayong (December 2006). "Timing of recovery from the end-Permian extinction: Geochronologic and biostratigraphic constraints from south China". Geology 34 (12): 1053–1056. Bibcode:2006Geo....34.1053L. doi:10.1130/G22827A.1.
Yadong
Sun1,2,*, Michael M. Joachimski3, Paul B. Wignall2, Chunbo Yan1,
Yanlong Chen4, Haishui Jiang1, Lina Wang1, Xulong Lai1 (2012). "Lethally
Hot Temperatures During the Early Triassic Greenhouse". Science 338 (6105): 366–370. Bibcode:2012Sci...338..366S. doi:10.1126/science.1224126. PMID 23087244.
During the greatest mass extinction in Earth’s history the world’s oceans reached 40 °C (104 °F) – lethally hot.
Lau,
Kimberly V.; Maher, Kate; Altiner, Demir; Kelley, Brian M.; Kump, Lee
R.; Lehrmann, Daniel J.; Silva-Tamayo, Juan Carlos; Weaver, Karrie L.;
Yu, Meiyi; Payne, Jonathan L. (2016). "Marine anoxia and delayed Earth system recovery after the end-Permian extinction". Proceedings of the National Academy of Sciences 113 (9): 2360–2365. doi:10.1073/pnas.1515080113.
Ward
PD, Montgomery DR, & Smith R (2000). "Altered river morphology in
South Africa related to the Permian–Triassic extinction". Science 289 (5485): 1740–1743. Bibcode:2000Sci...289.1740W. doi:10.1126/science.289.5485.1740. PMID 10976065.
Hallam, A; Wignall, P B (1997). Mass Extinctions and their Aftermath. Oxford University Press. ISBN 978-0-19-854916-1.
Rodland,
DL & Bottjer, DJ (2001). "Biotic Recovery from the End-Permian Mass
Extinction: Behavior of the Inarticulate Brachiopod Lingula as a Disaster Taxon". PALAIOS 16 (1): 95–101. doi:10.1669/0883-1351(2001)016<0095:brftep>2.0.CO;2. ISSN 0883-1351.
Zi-qiang W (1996). "Recovery of vegetation from the terminal Permian mass extinction in North China". Review of Palaeobotany and Palynology 91 (1–4): 121–142. doi:10.1016/0034-6667(95)00069-0.
Wagner
PJ, Kosnik MA, & Lidgard S (2006). "Abundance Distributions Imply
Elevated Complexity of Post-Paleozoic Marine Ecosystems". Science 314 (5803): 1289–1292. Bibcode:2006Sci...314.1289W. doi:10.1126/science.1133795. PMID 17124319.
Clapham, M.E., Bottjer, D.J. and Shen, S. (2006). "Decoupled diversity and ecology during the end-Guadalupian extinction (late Permian)". Geological Society of America Abstracts with Programs 38 (7): 117. Retrieved 2008-03-28.
Baumiller, T. K. (2008). "Crinoid Ecological Morphology". Annual Review of Earth and Planetary Sciences 36 (1): 221–249. Bibcode:2008AREPS..36..221B. doi:10.1146/annurev.earth.36.031207.124116.
Botha, J., and Smith, R.M.H. (2007). "Lystrosaurus species composition across the Permo–Triassic boundary in the Karoo Basin of South Africa". Lethaia 40 (2): 125–137. doi:10.1111/j.1502-3931.2007.00011.x. Retrieved 2008-07-02. Full version online at "Lystrosaurus species composition across the Permo–Triassic boundary in the Karoo Basin of South Africa" (PDF). Retrieved 2008-07-02.
Benton, M.J. (2004). Vertebrate Paleontology. Blackwell Publishers. xii–452. ISBN 0-632-05614-2.
Ruben, J.A., and Jones, T.D. (2000). "Selective Factors Associated with the Origin of Fur and Feathers". American Zoologist 40 (4): 585–596. doi:10.1093/icb/40.4.585.
Yates AM & Warren AA (2000). "The
phylogeny of the 'higher' temnospondyls (Vertebrata: Choanata) and its
implications for the monophyly and origins of the Stereospondyli". Zoological Journal of the Linnean Society 128 (1): 77–121. doi:10.1111/j.1096-3642.2000.tb00650.x. Archived from the original on 2007-10-01. Retrieved 2008-01-18.
Retallack GJ, Seyedolali A, Krull ES, Holser WT, Ambers CP, Kyte FT (1998). "Search for evidence of impact at the Permian–Triassic boundary in Antarctica and Australia". Geology 26 (11): 979–982. Bibcode:1998Geo....26..979R. doi:10.1130/0091-7613(1998)026<0979:sfeoia>2.3.CO;2.
Becker
L, Poreda RJ, Basu AR, Pope KO, Harrison TM, Nicholson C, Iasky R
(2004). "Bedout: a possible end-Permian impact crater offshore of
northwestern Australia". Science 304 (5676): 1469–1476. Bibcode:2004Sci...304.1469B. doi:10.1126/science.1093925. PMID 15143216.
Becker
L, Poreda RJ, Hunt AG, Bunch TE, Rampino M (2001). "Impact event at the
Permian–Triassic boundary: Evidence from extraterrestrial noble gases
in fullerenes". Science 291 (5508): 1530–1533. Bibcode:2001Sci...291.1530B. doi:10.1126/science.1057243. PMID 11222855.
Basu
AR, Petaev MI, Poreda RJ, Jacobsen SB, Becker L (2003). "Chondritic
meteorite fragments associated with the Permian–Triassic boundary in
Antarctica". Science 302 (5649): 1388–1392. Bibcode:2003Sci...302.1388B. doi:10.1126/science.1090852. PMID 14631038.
Kaiho K, Kajiwara Y, Nakano T, Miura Y, Kawahata H, Tazaki K, Ueshima M, Chen Z, Shi GR (2001). "End-Permian catastrophe by a bolide impact: Evidence of a gigantic release of sulfur from the mantle". Geology 29 (9): 815–818. Bibcode:2001Geo....29..815K. doi:10.1130/0091-7613(2001)029<0815:epcbab>2.0.CO;2. ISSN 0091-7613. Retrieved 2007-10-22.
Farley
KA, Mukhopadhyay S, Isozaki Y, Becker L, Poreda RJ (2001). "An
extraterrestrial impact at the Permian–Triassic boundary?". Science 293 (5539): 2343a–2343. doi:10.1126/science.293.5539.2343a. PMID 11577203.
Koeberl
C, Gilmour I, Reimold WU, Philippe Claeys P, Ivanov B (2002).
"End-Permian catastrophe by bolide impact: Evidence of a gigantic
release of sulfur from the mantle: Comment and Reply". Geology 30 (9): 855–856. Bibcode:2002Geo....30..855K. doi:10.1130/0091-7613(2002)030<0855:epcbbi>2.0.CO;2. ISSN 0091-7613.
Isbell
JL, Askin RA, Retallack GR (1999). "Search for evidence of impact at
the Permian–Triassic boundary in Antarctica and Australia; discussion
and reply". Geology 27 (9): 859–860. Bibcode:1999Geo....27..859I. doi:10.1130/0091-7613(1999)027<0859:sfeoia>2.3.CO;2.
Koeberl
K, Farley KA, Peucker-Ehrenbrink B, Sephton MA (2004). "Geochemistry of
the end-Permian extinction event in Austria and Italy: No evidence for
an extraterrestrial component". Geology 32 (12): 1053–1056. Bibcode:2004Geo....32.1053K. doi:10.1130/G20907.1.
Langenhorst F, Kyte FT & Retallack GJ (2005). "Reexamination of quartz grains from the Permian–Triassic boundary section at Graphite Peak, Antarctica" (PDF). Lunar and Planetary Science Conference XXXVI. Retrieved 2007-07-13.
Jones
AP, Price GD, Price NJ, DeCarli PS, Clegg RA (2002). "Impact induced
melting and the development of large igneous provinces". Earth and Planetary Science Letters 202 (3): 551–561. Bibcode:2002E&PSL.202..551J. doi:10.1016/S0012-821X(02)00824-5.
White RV (2002). "Earth's biggest 'whodunnit': unravelling the clues in the case of the end-Permian mass extinction" (PDF). Phil. Trans. Royal Society of London 360 (1801): 2963–2985. Bibcode:2002RSPTA.360.2963W. doi:10.1098/rsta.2002.1097. PMID 12626276. Retrieved 2008-01-12.
AHager, Bradford H (2001). "Giant Impact Craters Lead To Flood Basalts: A Viable Model". CCNet 33/2001: Abstract 50470.
Hagstrum, Jonathan T (2001). "Large Oceanic Impacts As The Cause Of Antipodal Hotspots And Global Mass Extinctions". CCNet 33/2001: Abstract 50288.
von Frese RR, Potts L, Gaya-Pique L, Golynsky AV, Hernandez O, Kim J, Kim H & Hwang J (2006). "Permian–Triassic mascon in Antarctica". Eos Trans. AGU, Jt. Assem. Suppl. 87 (36): Abstract T41A–08. Retrieved 2007-10-22.
Von
Frese, R.R.B.; L. V. Potts; S. B. Wells; T. E. Leftwich; H. R. Kim; J.
W. Kim; A. V. Golynsky; O. Hernandez; L. R. Gaya-Piqué (2009). "GRACE
gravity evidence for an impact basin in Wilkes Land, Antarctica". Geochem. Geophys. Geosyst. 10 (2): Q02014. Bibcode:2009GGG....1002014V. doi:10.1029/2008GC002149.
Tohver
E., Lana C., Cawood P.A., Fletcher I.R., Jourdan F., Sherlock S.,
Rasmussen B., Trindade R.I.F., Yokoyama E., Filho C.R. Souza, Marangoni
Y. "Geochronological
constraints on the age of a Permo–Triassic impact event: U–Pb and
40Ar/39Ar results for the 40 km Araguainha structure of central Brazil". Geochimica et Cosmochimica Acta 86: 214–227. Bibcode:2012GeCoA..86..214T. doi:10.1016/j.gca.2012.03.005.
Biggest
extinction in history caused by climate-changing meteor. University of
Western Australia University News Wednesday, 31 July 2013. http://www.news.uwa.edu.au/201307315921/international/biggest-extinction-history-caused-climate-changing-meteor
Zhou,
M-F., Malpas, J, Song, X-Y, Robinson, PT, Sun, M, Kennedy, AK, Lesher,
CM & Keays, RR (2002). "A temporal link between the Emeishan large
igneous province (SW China) and the end-Guadalupian mass extinction". Earth and Planetary Science Letters 196 (3–4): 113–122. Bibcode:2002E&PSL.196..113Z. doi:10.1016/S0012-821X(01)00608-2.
Wignall, Paul B.; et al. (2009). "Volcanism, Mass Extinction, and Carbon Isotope Fluctuations in the Middle Permian of China". Science 324 (5931): 1179–1182. Bibcode:2009Sci...324.1179W. doi:10.1126/science.1171956. PMID 19478179.
Andy Saunders, Marc Reichow (2009). "The Siberian Traps – Area and Volume". Retrieved 2009-10-18.
Andy Saunders and Marc Reichow (January 2009). "The Siberian Traps and the End-Permian mass extinction: a critical review" (PDF). Chinese Science Bulletin (Springer) 54 (1): 20–37. doi:10.1007/s11434-008-0543-7.
Reichow,
MarcK.; Pringle, M.S.; Al'Mukhamedov, A.I.; Allen, M.B.; Andreichev,
V.L.; Buslov, M.M.; Davies, C.E.; Fedoseev, G.S.; Fitton, J.G.; Inger,
S.; Medvedev, A.Ya.; Mitchell, C.; Puchkov, V.N.; Safonova, I.Yu.;
Scott, R.A.; Saunders, A.D. (2009). "The
timing and extent of the eruption of the Siberian Traps large igneous
province: Implications for the end-Permian environmental crisis" (PDF). Earth and Planetary Science Letters 277: 9–20. Bibcode:2009E&PSL.277....9R. doi:10.1016/j.epsl.2008.09.030.
Kamo, SL (2003). "Rapid
eruption of Siberian flood-volcanic rocks and evidence for coincidence
with the Permian–Triassic boundary and mass extinction at 251 Ma". Earth and Planetary Science Letters 214: 75–91. Bibcode:2003E&PSL.214...75K. doi:10.1016/S0012-821X(03)00347-9.
"Volcanism".
Dan Verango (January 24, 2011). "Ancient mass extinction tied to torched coal". USA Today.
Stephen E. Grasby, Hamed Sanei & Benoit Beauchamp (January 23, 2011). "Catastrophic dispersion of coal fly ash into oceans during the latest Permian extinction". Nature Geoscience 4 (2): 104–107. Bibcode:2011NatGe...4..104G. doi:10.1038/ngeo1069.
"Researchers
find smoking gun of world's biggest extinction; Massive volcanic
eruption, burning coal and accelerated greenhouse gas choked out life". University of Calgary. January 23, 2011. Retrieved 2011-01-26.
Yang,
QY (2013). "The chemical compositions and abundances of volatiles in
the Siberian large igneous province: Constraints on magmatic CO2 and SO2
emissions into the atmosphere". Chemical Geology 339: 84–91. doi:10.1016/j.chemgeo.2012.08.031.
Burgess, Seth D.; Bowring, Samuel; Shen, Shu-zhong (2014-03-04). "High-precision timeline for Earth’s most severe extinction". Proceedings of the National Academy of Sciences 111 (9): 3316–3321. doi:10.1073/pnas.1317692111. ISSN 0027-8424. PMC 3948271. PMID 24516148.
"Earth’s worst extinction "inescapably" tied to Siberian Traps, CO2, and climate change". Skeptical Science. Retrieved 2016-03-11.
Black, Benjamin A.; Weiss, Benjamin P.; Elkins-Tanton, Linda T.; Veselovskiy, Roman V.; Latyshev, Anton (2015-04-30). "Siberian Traps volcaniclastic rocks and the role of magma-water interactions". Geological Society of America Bulletin 127: B31108.1. doi:10.1130/B31108.1. ISSN 0016-7606.
Burgess, Seth D.; Bowring, Samuel A. (2015-08-01). "High-precision geochronology confirms voluminous magmatism before, during, and after Earth’s most severe extinction". Science Advances 1 (7): e1500470. doi:10.1126/sciadv.1500470. ISSN 2375-2548. PMC 4643808. PMID 26601239.
Fischman, Josh. "Giant Eruptions and Giant Extinctions [Video]". Scientific American. Retrieved 2016-03-11.
Palfy
J, Demeny A, Haas J, Htenyi M, Orchard MJ, & Veto I (2001). "Carbon
isotope anomaly at the Triassic– Jurassic boundary from a marine
section in Hungary". Geology 29 (11): 1047–1050. Bibcode:2001Geo....29.1047P. doi:10.1130/0091-7613(2001)029<1047:ciaaog>2.0.CO;2. ISSN 0091-7613.
Berner, R.A. (2002). "Examination of hypotheses for the Permo-Triassic boundary extinction by carbon cycle modeling". Proceedings of the National Academy of Sciences 99 (7): 4172–4177. Bibcode:2002PNAS...99.4172B. doi:10.1073/pnas.032095199. PMC 123621. PMID 11917102.
Dickens
GR, O'Neil JR, Rea DK & Owen RM (1995). "Dissociation of oceanic
methane hydrate as a cause of the carbon isotope excursion at the end of
the Paleocene". Paleoceanography 10 (6): 965–71. Bibcode:1995PalOc..10..965D. doi:10.1029/95PA02087.
White, R. V. (2002). "Earth's biggest 'whodunnit': Unravelling the clues in the case of the end-Permian mass extinction". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 360 (1801): 2963–85. doi:10.1098/rsta.2002.1097. PMID 12626276.
Schrag, D.P., Berner, R.A., Hoffman, P.F., and Halverson, G.P. (2002). "On the initiation of a snowball Earth". Geochemistry Geophysics Geosystems 3 (6): 1036. Bibcode:2002GGG....3fQ...1S. doi:10.1029/2001GC000219. Preliminary abstract at Schrag, D.P. (June 2001). "On the initiation of a snowball Earth". Geological Society of America.
Benton, M.J.; Twitchett, R.J. (2003). "How to kill (almost) all life: the end-Permian extinction event". Trends in Ecology & Evolution 18 (7): 358–365. doi:10.1016/S0169-5347(03)00093-4.
Dickens GR (2001). "The potential volume of oceanic methane hydrates with variable external conditions". Organic Geochemistry 32 (10): 1179–1193. doi:10.1016/S0146-6380(01)00086-9.
Reichow MK, Saunders AD, White RV, Pringle MS, Al'Muhkhamedov AI, Medvedev AI & Kirda NP (2002). "40Ar/39Ar Dates from the West Siberian Basin: Siberian Flood Basalt Province Doubled". Science 296 (5574): 1846–1849. Bibcode:2002Sci...296.1846R. doi:10.1126/science.1071671. PMID 12052954.
Holser
WT, Schoenlaub H-P, Attrep Jr M, Boeckelmann K, Klein P, Magaritz M,
Orth CJ, Fenninger A, Jenny C, Kralik M, Mauritsch H, Pak E, Schramm
J-F, Stattegger K & Schmoeller R (1989). "A unique geochemical
record at the Permian/Triassic boundary". Nature 337 (6202): 39–44. Bibcode:1989Natur.337...39H. doi:10.1038/337039a0.
Dobruskina IA (1987). "Phytogeography of Eurasia during the early Triassic". Palaeogeography, Palaeoclimatology, Palaeoecology 58 (1–2): 75–86. doi:10.1016/0031-0182(87)90007-1.
Wignall, P.B.; Twitchett, R.J. (2002). "Extent, duration, and nature of the Permian-Triassic superanoxic event". Geological Society of America Special Papers 356: 395–413. doi:10.1130/0-8137-2356-6.395. ISBN 0-8137-2356-6.
Cao,
Changqun; Gordon D. Love; Lindsay E. Hays; Wei Wang; Shuzhong Shen;
Roger E. Summons (2009). "Biogeochemical evidence for euxinic oceans and
ecological disturbance presaging the end-Permian mass extinction
event". Earth and Planetary Science Letters 281 (3–4): 188–201. Bibcode:2009E&PSL.281..188C. doi:10.1016/j.epsl.2009.02.012.
Hays,
Lindsay; Kliti Grice; Clinton B. Foster; Roger E. Summons (2012).
"Biomarker and isotopic trends in a Permian–Triassic sedimentary section
at Kap Stosch, Greenland". Organic Geochemistry 43: 67–82. doi:10.1016/j.orggeochem.2011.10.010.
Meyers,
Katja; L.R. Kump; A. Ridgwell (September 2008). "Biogeochemical
controls on photic-zone euxinia during the end-Permian mass extinction".
Geology 36 (9): 747–750. doi:10.1130/G24618A (inactive 2016-03-11).
Kump,
Lee; Alexander Pavlov; Michael A. Arthur (2005). "Massive release of
hydrogen sulfide to the surface ocean and atmosphere during intervals of
oceanic anoxia". Geology 33 (5): 397–400. Bibcode:2005Geo....33..397K. doi:10.1130/G21295.1.
The Permian – Palaeos
Rothman, D. H.; Fournier, G. P.; French, K. L.; Alm, E. J.; Boyle, E. A.; Cao, C.; Summons, R. E. (2014-03-31). "Methanogenic burst in the end-Permian carbon cycle". Proceedings of the National Academy of Sciences 111 (15): 5462–7. doi:10.1073/pnas.1318106111. PMC 3992638. PMID 24706773. — Lay summary: Chandler, David L.; Massachusetts Institute of Technology (March 31, 2014). "Ancient whodunit may be solved: Methane-producing microbes did it!". Science Daily.
Shen, Shu-Zhong; Bowring, Samuel A. (2014). "The end-Permian mass extinction: A still unexplained catastrophe". National Science Review 1 (4): 492–495. doi:10.1093/nsr/nwu047.
 ENLARGE
ENLARGE