Obviously, meaning number 2 best describes the sun plasma.
Dictionary
plas·ma
ˈplazmə/
noun
noun: plasma; noun: plasm
- 1.the colorless fluid part of blood, lymph, or milk, in which corpuscles or fat globules are suspended.
- 2.an ionized gas consisting of positive ions and free electrons in proportions resulting in more or less no overall electric charge, typically at low pressures (as in the upper atmosphere and in fluorescent lamps) or at very high temperatures (as in stars and nuclear fusion reactors).
- an analogous substance consisting of mobile charged particles (such as a molten salt or the electrons within a metal).
- 3.a dark green, translucent variety of quartz used in mosaic and for other decorative purposes.
- 4.another term for cytoplasm or protoplasm.
Origin
early 18th century (in the sense ‘mold, shape’): from late Latin, literally ‘mold,’ from Greek plasma, from plassein ‘to shape.’
Translate plasma to
What is Plasma? | CSL Plasma
https://www.cslplasma.com/what-is-plasma
Plasma is about 90% water and it makes up 55% of blood volume. Plasma
contains cells, nutrients, proteins, enzymes, hormones, salts and more.
Its primary objective is to move and transport material throughout the
body to areas that are in need.
It is my understanding presently that plasma mostly exists here on earth as lightning. We don't naturally see plasma in many other forms outside of lightning. However, in a star like our sun plasma exists as a form of hydrogen bombs going off all the time which gives the light we see through space from our sun. So, maybe thinking of the sun as a Sea of Hydrogen plasma slowly turning into Helium and other elements might be the best way "in regard to physics" to actually see our sun and other stars. My understanding is Hydrogen through a fusion reaction converts to helium and other heavier elements through a fusion process similar or exactly the same as a fusion hydrogen bomb uses when tested here on earth.
begin quote from:
Plasma (physics) - Wikipedia
https://en.wikipedia.org/wiki/Plasma_(physics)
  |
|
  |
|
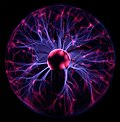
| Top: Lightning and neon lights are commonplace generators of plasma. Bottom left: A plasma globe, illustrating some of the more complex plasma phenomena, including filamentation. Bottom right: A plasma trail from the Space Shuttle Atlantis during re-entry into Earth's atmosphere, as seen from the International Space Station. |
Plasma and ionised gases have unique properties and display behaviors unlike those of the other states, although the true technical transition between the two is mostly a matter of nomenclature[2] and subject to interpretation.[5] It can simply be considered as a gaseous mixture of negatively charged electrons and highly charged positive ions, being created by heating a gas or by subjecting gas to a strong electromagnetic field. However, true plasma production is from the distinct separation of these ions and electrons that produces an electric field, which in turn, produces electric currents and magnetic fields.
Based on the surrounding environmental temperature and density either partially ionised or fully ionised forms of plasma may be produced. Partially ionised plasma is popularly understood, for example, as bright neon signs or lightning storms,[6] while more fully ionised plasma is associated with the interior of the Sun,[7] the solar corona,[8] and stars.[9]
The positive charge in ions is achieved by stripping away electrons from atomic nuclei. The number of electrons removed is related to either the increase in temperature or the local density of other ionised matter. This also can be accompanied by the dissociation of molecular bonds,[10] though this fundamental process is distinctly different from chemical processes of ion interactions in liquids or the behaviour of ions existing in metals. A significant number of highly charged particles together make plasma electrically conductive so that it responds strongly to electromagnetic fields, and this property can be usefully employed in many modern technological devices, such as, plasma televisions or plasma etching.[11]
Plasma may be the most abundant form of ordinary matter in the universe,[12] although this is currently tentative based on the existence and unknown properties of dark matter. Plasma is mostly associated with stars, extending to the rarefied intracluster medium and possibly the intergalactic regions.[13]
Contents
History
Plasma was first identified in a Crookes tube, and so described by Sir William Crookes in 1879 (he called it "radiant matter").[14] The nature of the Crookes tube "cathode ray" matter was subsequently identified by British physicist Sir J.J. Thomson in 1897.[15] The term "plasma" was coined by Irving Langmuir in 1928,[16] perhaps because the glowing discharge molds itself to the shape of the Crookes tube (Gr. πλάσμα – a thing moulded or formed).[17] Langmuir described his observations as:- "Except near the electrodes, where there are sheaths containing very few electrons, the ionized gas contains ions and electrons in about equal numbers so that the resultant space charge is very small. We shall use the name plasma to describe this region containing balanced charges of ions and electrons."[16]
| Continuum mechanics |
|---|
Properties and parameters
Artist's rendition of the Earth's plasma fountain,
showing oxygen, helium, and hydrogen ions that gush into space from
regions near the Earth's poles. The faint yellow area shown above the
north pole represents gas lost from Earth into space; the green area is
the aurora borealis, where plasma energy pours back into the atmosphere.[18]
Definition
Plasma is an electrically neutral medium of unbound positive and negative particles (i.e. the overall charge of a plasma is roughly zero). Although these particles are unbound, they are not ‘free’ in the sense of not experiencing forces. Moving charged particles generate an electric current within a magnetic field; and any movement of a charged plasma particle affects and is affected by the general field created by the motion of other charges. In turn this governs collective behavior with many degrees of variation.[10][19] Three factors are listed in the definition of a plasma stream:[20][21]- The plasma approximation: Charged particles must be close enough together that each particle influences many nearby charged particles, rather than just interacting with the closest particle (these collective effects are a distinguishing feature of a plasma).[citation needed] The plasma approximation is valid when the number of charge carriers within the sphere of influence (called the Debye sphere whose radius is the Debye screening length) of a particular particle is higher than unity to provide collective behavior of the charged particles. The average number of particles in the Debye sphere is given by the plasma parameter,[ambiguous] "Λ" (the Greek uppercase letter Lambda).[citation needed]
- Bulk interactions: The Debye screening length (defined above) is short compared to the physical size of the plasma. This criterion means that interactions in the bulk of the plasma are more important than those at its edges, where boundary effects may take place. When this criterion is satisfied, the plasma is quasineutral.[citation needed]
- Plasma frequency: The electron plasma frequency (measuring plasma oscillations of the electrons) is large compared to the electron-neutral collision frequency (measuring frequency of collisions between electrons and neutral particles). When this condition is valid, electrostatic interactions dominate over the processes of ordinary gas kinetics.[citation needed]
Degree of ionization
For plasma to exist, ionization is necessary. The term "plasma density" by itself usually refers to the "electron density", that is, the number of free electrons per unit volume. The degree of ionization of a plasma is the proportion of atoms that have lost or gained electrons, and is controlled mostly by the temperature. Even a partially ionized gas in which as little as 1% of the particles are ionized can have the characteristics of a plasma (i.e., response to magnetic fields and high electrical conductivity). The degree of ionization, , is defined as
, is defined as  , where
, where  is the number density of ions and
is the number density of ions and  is the number density of neutral atoms. The electron density is related to this by the average charge state
is the number density of neutral atoms. The electron density is related to this by the average charge state  of the ions through
of the ions through  , where
, where  is the number density of electrons.
is the number density of electrons.Temperatures
Plasma temperature is commonly measured in kelvins or electronvolts and is, informally, a measure of the thermal kinetic energy per particle. High temperatures are usually needed to sustain ionization, which is a defining feature of a plasma. The degree of plasma ionization is determined by the electron temperature relative to the ionization energy (and more weakly by the density), in a relationship called the Saha equation. At low temperatures, ions and electrons tend to recombine into bound states—atoms[22]—and the plasma will eventually become a gas.In most cases the electrons are close enough to thermal equilibrium that their temperature is relatively well-defined, even when there is a significant deviation from a Maxwellian energy distribution function, for example, due to UV radiation, energetic particles, or strong electric fields. Because of the large difference in mass, the electrons come to thermodynamic equilibrium amongst themselves much faster than they come into equilibrium with the ions or neutral atoms. For this reason, the ion temperature may be very different from (usually lower than) the electron temperature. This is especially common in weakly ionized technological plasmas, where the ions are often near the ambient temperature.
Thermal vs. nonthermal plasmas
Based on the relative temperatures of the electrons, ions and neutrals, plasmas are classified as "thermal" or "non-thermal". Thermal plasmas have electrons and the heavy particles at the same temperature, i.e. they are in thermal equilibrium with each other. Nonthermal plasmas on the other hand have the ions and neutrals at a much lower temperature (sometimes room temperature), whereas electrons are much "hotter" ( ).[23]
).[23]Complete vs. incomplete ionization
A plasma is sometimes referred to as being "hot" if it is nearly fully ionized, or "cold" if only a small fraction (for example 1%) of the gas molecules are ionized, but other definitions of the terms "hot plasma" and "cold plasma" are common. Even in a "cold" plasma, the electron temperature is still typically several thousand degrees Celsius. Plasmas utilized in "plasma technology" ("technological plasmas") are usually cold plasmas in the sense that only a small fraction of the gas molecules are ionized.[citation needed]Plasma potential
Lightning
is an example of plasma present at Earth's surface. Typically,
lightning discharges 30,000 amperes at up to 100 million volts, and
emits light, radio waves, X-rays and even gamma rays.[24] Plasma temperatures in lightning can approach 28,000 K (28,000 °C; 50,000 °F) and electron densities may exceed 1024 m−3.
 ), but on the scale of the Debye length there can be charge imbalance. In the special case that double layers are formed, the charge separation can extend some tens of Debye lengths.[citation needed]
), but on the scale of the Debye length there can be charge imbalance. In the special case that double layers are formed, the charge separation can extend some tens of Debye lengths.[citation needed]The magnitude of the potentials and electric fields must be determined by means other than simply finding the net charge density. A common example is to assume that the electrons satisfy the Boltzmann relation:
In astrophysical plasmas, Debye screening prevents electric fields from directly affecting the plasma over large distances, i.e., greater than the Debye length. However, the existence of charged particles causes the plasma to generate, and be affected by, magnetic fields. This can and does cause extremely complex behavior, such as the generation of plasma double layers, an object that separates charge over a few tens of Debye lengths. The dynamics of plasmas interacting with external and self-generated magnetic fields are studied in the academic discipline of magnetohydrodynamics.[citation needed]
Magnetization
Plasma with a magnetic field strong enough to influence the motion of the charged particles is said to be magnetized. A common quantitative criterion is that a particle on average completes at least one gyration around the magnetic field before making a collision, i.e., , where
, where  is the "electron gyrofrequency" and
is the "electron gyrofrequency" and  is the "electron collision rate". It is often the case that the
electrons are magnetized while the ions are not. Magnetized plasmas are anisotropic,
meaning that their properties in the direction parallel to the magnetic
field are different from those perpendicular to it. While electric
fields in plasmas are usually small due to the high conductivity, the
electric field associated with a plasma moving in a magnetic field is
given by
is the "electron collision rate". It is often the case that the
electrons are magnetized while the ions are not. Magnetized plasmas are anisotropic,
meaning that their properties in the direction parallel to the magnetic
field are different from those perpendicular to it. While electric
fields in plasmas are usually small due to the high conductivity, the
electric field associated with a plasma moving in a magnetic field is
given by  (where
(where  is the electric field,
is the electric field,  is the velocity, and
is the velocity, and  is the magnetic field), and is not affected by Debye shielding.[25]
is the magnetic field), and is not affected by Debye shielding.[25]Comparison of plasma and gas phases
Plasma is often called the fourth state of matter after solid, liquids and gases, despite plasma typically being an ionized gas.[26][27] It is distinct from these and other lower-energy states of matter. Although it is closely related to the gas phase in that it also has no definite form or volume, it differs in a number of ways, including the following:| Property | Gas | Plasma |
|---|---|---|
| Electrical conductivity | Very low: Air is an excellent insulator until it breaks down into plasma at electric field strengths above 30 kilovolts per centimeter.[28] | Usually very high: For many purposes, the conductivity of a plasma may be treated as infinite. |
| Independently acting species | One: All gas particles behave in a similar way, influenced by gravity and by collisions with one another. | Two or three: Electrons, ions, protons and neutrons can be distinguished by the sign and value of their charge so that they behave independently in many circumstances, with different bulk velocities and temperatures, allowing phenomena such as new types of waves and instabilities. |
| Velocity distribution | Maxwellian: Collisions usually lead to a Maxwellian velocity distribution of all gas particles, with very few relatively fast particles. | Often non-Maxwellian: Collisional interactions are often weak in hot plasmas and external forcing can drive the plasma far from local equilibrium and lead to a significant population of unusually fast particles. |
| Interactions | Binary: Two-particle collisions are the rule, three-body collisions extremely rare. | Collective: Waves, or organized motion of plasma, are very important because the particles can interact at long ranges through the electric and magnetic forces. |
Plasmas in astronomy and astrophysics
Plasmas are by far the most common phase of ordinary matter in the universe, both by mass and by volume.[29] Most of the visible light from space from the created electromagnetic radiation produced either the plasma confined within stars or hot regions in the intergalactic medium that radiate strongly in X-rays.[citation needed]Within our Solar System, interplanetary space is filled with the plasma expelled via the solar wind, extending from the Sun's surface out to the heliopause. Furthermore, all the distant stars, and much of interstellar space or intergalactic space is also likely filled with plasma, albeit at very low densities. Astrophysical plasmas are also observed in Accretion disks around stars or compact objects like white dwarfs, neutron stars, or black holes in close binary star systems.[30] Plasma is associated with ejection of material in astrophysical jets, which have been observed with accreting black holes[31] or in active galaxies like M87's jet that possibly extends out to 5,000 light-years.[32]
Common plasmas
Plasmas can appear in Nature in various forms and locations, which can be usefully broadly summarised in the following Table:| Artificially produced | Terrestrial plasmas | Space and astrophysical plasmas |
|---|---|---|
|
|
|
Complex plasma phenomena
Although the underlying equations governing plasmas are relatively simple, plasma behavior is extraordinarily varied and subtle: the emergence of unexpected behavior from a simple model is a typical feature of a complex system. Such systems lie in some sense on the boundary between ordered and disordered behavior and cannot typically be described either by simple, smooth, mathematical functions, or by pure randomness. The spontaneous formation of interesting spatial features on a wide range of length scales is one manifestation of plasma complexity. The features are interesting, for example, because they are very sharp, spatially intermittent (the distance between features is much larger than the features themselves), or have a fractal form. Many of these features were first studied in the laboratory, and have subsequently been recognized throughout the universe. Examples of complexity and complex structures in plasmas include:Filamentation
Striations or string-like structures,[34] also known as Birkeland currents, are seen in many plasmas, like the plasma ball, the aurora,[35] lightning,[36] electric arcs, solar flares,[37] and supernova remnants.[38] They are sometimes associated with larger current densities, and the interaction with the magnetic field can form a magnetic rope structure.[39] High power microwave breakdown at atmospheric pressure also leads to the formation of filamentary structures.[40] (See also Plasma pinch)Filamentation also refers to the self-focusing of a high power laser pulse. At high powers, the nonlinear part of the index of refraction becomes important and causes a higher index of refraction in the center of the laser beam, where the laser is brighter than at the edges, causing a feedback that focuses the laser even more. The tighter focused laser has a higher peak brightness (irradiance) that forms a plasma. The plasma has an index of refraction lower than one, and causes a defocusing of the laser beam. The interplay of the focusing index of refraction, and the defocusing plasma makes the formation of a long filament of plasma that can be micrometers to kilometers in length.[41] One interesting aspect of the filamentation generated plasma is the relatively low ion density due to defocusing effects of the ionized electrons.[42] (See also Filament propagation)
Non-neutral plasma
The strength and range of the electric force and the good conductivity of plasmas usually ensure that the densities of positive and negative charges in any sizeable region are equal ("quasineutrality"). A plasma with a significant excess of charge density, or, in the extreme case, is composed of a single species, is called a non-neutral plasma. In such a plasma, electric fields play a dominant role. Examples are charged particle beams, an electron cloud in a Penning trap and positron plasmas.[43]Dusty plasma/grain plasma
A dusty plasma contains tiny charged particles of dust (typically found in space). The dust particles acquire high charges and interact with each other. A plasma that contains larger particles is called grain plasma. Under laboratory conditions, dusty plasmas are also called complex plasmas.[44]Impermeable plasma
Impermeable plasma is a type of thermal plasma which acts like an impermeable solid with respect to gas or cold plasma and can be physically pushed. Interaction of cold gas and thermal plasma was briefly studied by a group led by Hannes Alfvén in 1960s and 1970s for its possible applications in insulation of fusion plasma from the reactor walls.[45] However, later it was found that the external magnetic fields in this configuration could induce kink instabilities in the plasma and subsequently lead to an unexpectedly high heat loss to the walls.[46] In 2013, a group of materials scientists reported that they have successfully generated stable impermeable plasma with no magnetic confinement using only an ultrahigh-pressure blanket of cold gas. While spectroscopic data on the characteristics of plasma were claimed to be difficult to obtain due to the high pressure, the passive effect of plasma on synthesis of different nanostructures clearly suggested the effective confinement. They also showed that upon maintaining the impermeability for a few tens of seconds, screening of ions at the plasma-gas interface could give rise to a strong secondary mode of heating (known as viscous heating) leading to different kinetics of reactions and formation of complex nanomaterials.[47]Mathematical descriptions
The complex self-constricting magnetic field lines and current paths in a field-aligned Birkeland current that can develop in a plasma.[48]
Fluid model
Fluid models describe plasmas in terms of smoothed quantities, like density and averaged velocity around each position (see Plasma parameters). One simple fluid model, magnetohydrodynamics, treats the plasma as a single fluid governed by a combination of Maxwell's equations and the Navier–Stokes equations. A more general description is the two-fluid plasma picture, where the ions and electrons are described separately. Fluid models are often accurate when collisionality is sufficiently high to keep the plasma velocity distribution close to a Maxwell–Boltzmann distribution. Because fluid models usually describe the plasma in terms of a single flow at a certain temperature at each spatial location, they can neither capture velocity space structures like beams or double layers, nor resolve wave-particle effects.Kinetic model
Kinetic models describe the particle velocity distribution function at each point in the plasma and therefore do not need to assume a Maxwell–Boltzmann distribution. A kinetic description is often necessary for collisionless plasmas. There are two common approaches to kinetic description of a plasma. One is based on representing the smoothed distribution function on a grid in velocity and position. The other, known as the particle-in-cell (PIC) technique, includes kinetic information by following the trajectories of a large number of individual particles. Kinetic models are generally more computationally intensive than fluid models. The Vlasov equation may be used to describe the dynamics of a system of charged particles interacting with an electromagnetic field. In magnetized plasmas, a gyrokinetic approach can substantially reduce the computational expense of a fully kinetic simulation.Artificial plasmas
Most artificial plasmas are generated by the application of electric and/or magnetic fields through a gas. Plasma generated in a laboratory setting and for industrial use can be generally categorized by:- The type of power source used to generate the plasma—DC, RF and microwave
- The pressure they operate at—vacuum pressure (< 10 mTorr or 1 Pa), moderate pressure (~ 1 Torr or 100 Pa), atmospheric pressure (760 Torr or 100 kPa)
- The degree of ionization within the plasma—fully, partially, or weakly ionized
- The temperature relationships within the plasma—thermal plasma (
), non-thermal or "cold" plasma (
)
- The electrode configuration used to generate the plasma
- The magnetization of the particles within the plasma—magnetized (both ion and electrons are trapped in Larmor orbits by the magnetic field), partially magnetized (the electrons but not the ions are trapped by the magnetic field), non-magnetized (the magnetic field is too weak to trap the particles in orbits but may generate Lorentz forces)[citation needed]
Generation of artificial plasma
Artificial plasma produced in air by a Jacob's Ladder
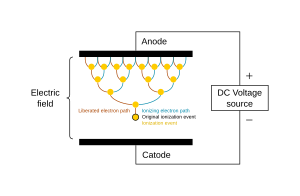
The potential difference and subsequent electric field pull the bound electrons (negative) toward the anode (positive electrode) while the cathode (negative electrode) pulls the nucleus.[50] As the voltage increases, the current stresses the material (by electric polarization) beyond its dielectric limit (termed strength) into a stage of electrical breakdown, marked by an electric spark, where the material transforms from being an insulator into a conductor (as it becomes increasingly ionized). The underlying process is the Townsend avalanche, where collisions between electrons and neutral gas atoms create more ions and electrons (as can be seen in the figure on the right). The first impact of an electron on an atom results in one ion and two electrons. Therefore, the number of charged particles increases rapidly (in the millions) only "after about 20 successive sets of collisions",[51] mainly due to a small mean free path (average distance travelled between collisions).
Electric arc
Cascade process of ionization. Electrons are ‘e−’, neutral atoms ‘o’, and cations ‘+’.
Avalanche effect between two electrodes. The original ionisation event
liberates one electron, and each subsequent collision liberates a
further electron, so two electrons emerge from each collision: the
ionising electron and the liberated electron.
Examples of industrial/commercial plasma
Because of their sizable temperature and density ranges, plasmas find applications in many fields of research, technology and industry. For example, in: industrial and extractive metallurgy,[52] surface treatments such as plasma spraying (coating), etching in microelectronics,[53] metal cutting[54] and welding; as well as in everyday vehicle exhaust cleanup and fluorescent/luminescent lamps,[49] while even playing a part in supersonic combustion engines for aerospace engineering.[55]Low-pressure discharges
- Glow discharge plasmas: non-thermal plasmas generated by the application of DC or low frequency RF (<100 a="" between="" class="mw-redirect" common="" electric="" electrodes.="" field="" gap="" generated="" href="https://en.wikipedia.org/wiki/Fluorescent_light" is="" khz="" metal="" most="" nbsp="" of="" plasma="" probably="" the="" this="" title="Fluorescent light" to="" two="" type="" within="">fluorescent light
Atmospheric pressure
- Arc discharge: this is a high power thermal discharge of very high temperature (~10,000 K). It can be generated using various power supplies. It is commonly used in metallurgical processes. For example, it is used to smelt minerals containing Al2O3 to produce aluminium.
- Corona discharge: this is a non-thermal discharge generated by the application of high voltage to sharp electrode tips. It is commonly used in ozone generators and particle precipitators.
- Dielectric barrier discharge (DBD): this is a non-thermal discharge generated by the application of high voltages across small gaps wherein a non-conducting coating prevents the transition of the plasma discharge into an arc. It is often mislabeled 'Corona' discharge in industry and has similar application to corona discharges. It is also widely used in the web treatment of fabrics.[60] The application of the discharge to synthetic fabrics and plastics functionalizes the surface and allows for paints, glues and similar materials to adhere.[61] The dielectric barrier discharge was used in the mid-1990s to show that low temperature atmospheric pressure plasma is effective in inactivating bacterial cells.[62] This work and later experiments using mammalian cells led to the establishment of a new field of research known as plasma medicine. The dielectric barrier discharge configuration was also used in the design of low temperature plasma jets. These plasma jets are produced by fast propagating guided ionization waves known as plasma bullets.[63]
- Capacitive discharge: this is a nonthermal plasma generated by the application of RF power (e.g., 13.56 MHz) to one powered electrode, with a grounded electrode held at a small separation distance on the order of 1 cm. Such discharges are commonly stabilized using a noble gas such as helium or argon.[64]
- "Piezoelectric direct discharge plasma:" is a nonthermal plasma generated at the high-side of a piezoelectric transformer (PT). This generation variant is particularly suited for high efficient and compact devices where a separate high voltage power supply is not desired.
Research
Plasmas are the object of study of the academic field of plasma science or plasma physics, including sub-disciplines such as space plasma physics. It currently involves the following fields of active research and features across many journals, whose interest includes:
|
|
Research examples
-
Hall effect thruster. The electric field in a plasma double layer is so effective at accelerating ions that electric fields are used in ion drives.
-
Solar plasma
See also
- Plasma torch
- Ambipolar diffusion
- Hannes Alfvén Prize
- Plasma channel
- Plasma parameters
- Plasma nitriding
- Magnetohydrodynamics (MHD)
- Electric field screening
- List of plasma physicists
- List of plasma (physics) articles
- Important publications in plasma physics
- IEEE Nuclear and Plasma Sciences Society
- Quark-gluon plasma
- Nikola Tesla
- Space physics
- Total electron content
- Plasma display
- Aurora
Notes
- Note that non-thermal, or non-equilibrium plasmas are not as ionized and have lower energy densities, and thus the temperature is not dispersed evenly among the particles, where some heavy ones remain ‘cold’.
References
- "High-tech dentistry – "St Elmo's frier" – Using a plasma torch to clean your teeth". The Economist print edition. Jun 17, 2009. Retrieved 2009-09-07.
External links
- Free plasma physics books and notes
- Plasmas: the Fourth State of Matter
- Plasma Science and Technology
- Plasma on the Internet – a list of plasma related links.
- Introduction to Plasma Physics: Graduate course given by Richard Fitzpatrick|M.I.T. Introduction by I.H.Hutchinson
- Plasma Material Interaction
- How to make a glowing ball of plasma in your microwave with a grape|More (Video)
- How to make plasma in your microwave with only one match (video)
- OpenPIC3D – 3D Hybrid Particle-In-Cell simulation of plasma dynamics
- Plasma Formulary Interactive











No comments:
Post a Comment