Desalination is the premier international journal dedicated to communicating the latest developments in ...
Desalination
From Wikipedia, the free encyclopedia
Desalination is a process that removes
minerals from saline
water. More generally, desalination refers to the removal of salts and minerals from a target substance,
[1] as in
soil desalination, which is an issue for agriculture.
[2]
Saltwater is desalinated to produce water suitable for
human consumption or
irrigation. One
by-product of desalination is
salt. Desalination is used on many seagoing
ships and
submarines. Most of the modern interest in desalination is focused on cost-effective provision of
fresh water for human use. Along with recycled
wastewater, it is one of the few rainfall-independent water sources.
[3]
Due to its energy consumption, desalinating sea water is generally more costly than fresh water from rivers or
groundwater,
water recycling and
water conservation.
However, these alternatives are not always available and depletion of
reserves is a critical problem worldwide. Currently, approximately 1% of
the world's population is dependent on desalinated water to meet daily
needs, but the UN expects that 14% of the world's population will
encounter water scarcity by 2025.
[4]
Desalination is particularly relevant in dry countries such as
Australia, which traditionally have relied on collecting rainfall behind dams for water.
According to the International Desalination Association, in June
2015, 18,426 desalination plants operated worldwide, producing 86.8
million cubic meters per day, providing water for 300 million people.
[5] This number increased from 78.4 million cubic meters in 2013,
[4] a 57% increase in just 5 years. The single largest desalination project is
Ras Al-Khair in
Saudi Arabia, which produced 1,025,000 cubic meters per day in 2014,
[4] although this plant is expected to be surpassed by a plant in California.
[6] Israel produces a higher proportion of its water than any other country, totaling 40% of its water use.
[7]
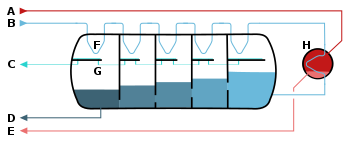
Schematic of a
multistage flash desalinator
A – steam in
B – seawater in
C – potable water out
D – waste out
E – steam out
F – heat exchange
G – condensation collection
H – brine heater
Methods
The traditional process used in these operations is
vacuum distillation—essentially
boiling it to leave impurities behind. In desalination, atmospheric
pressure is reduced, thus lowering the required temperature. Liquids
boil when the
vapor pressure
equals the ambient pressure and vapor pressure increases with
temperature. Thus, because of the reduced temperature, low-temperature
"waste" heat from electrical power generation or industrial processes
can be employed.
[citation needed]
Reverse osmosis desalination plant in Barcelona, Spain
The principal competing processes use membranes to desalinate, principally applying
reverse osmosis.
[8] Membrane processes use semipermeable membranes and pressure to separate salts from water.
Reverse osmosis plant
membrane systems typically use less energy than thermal distillation.
Desalination remains energy intensive, however, and future costs will
continue to depend on the energy prices.
[9]
Considerations and criticism
Energy consumption
Energy consumption of seawater desalination has reached as low as 3 kWh/m
3,
[10]
including pre-filtering and ancillaries, similar to the energy
consumption of other fresh water supplies transported over large
distances,
[11] but much higher than local fresh water supplies that use 0.2 kWh/m
3 or less.
[12]
A minimum energy consumption for seawater desalination of around 1 kWh/m
3 has been determined,
[13][14] excluding prefiltering and intake/outfall pumping. Under 2 kWh/m
3[15] has been achieved with
reverse osmosis membrane technology, leaving limited scope for further energy reductions.
Supplying all US domestic water by desalination would increase
energy consumption by around 10%, about the amount of energy used by domestic refrigerators.
[16] Domestic consumption is a relatively small fraction of the total water usage.
[17]
Note: "Electrical equivalent" refers to the amount of electrical
energy that could be generated using a given quantity of thermal energy
and appropriate turbine generator. These calculations do not include the
energy required to construct or refurbish items consumed in the
process.
Cogeneration
Cogeneration
is generating excess heat and electricity generation from a single
process. Cogeneration can provide usable heat for desalination in an
integrated, or "dual-purpose", facility where a power plant provides the
energy for desalination. Alternatively, the facility's energy
production may be dedicated to the production of potable water (a
stand-alone facility), or excess energy may be produced and incorporated
into the energy grid. Cogeneration takes various forms, and
theoretically any form of energy production could be used. However, the
majority of current and planned cogeneration desalination plants use
either
fossil fuels or
nuclear power as their source of energy. Most plants are located in the
Middle East or
North Africa,
which use their petroleum resources to offset limited water resources.
The advantage of dual-purpose facilities is they can be more efficient
in energy consumption, thus making desalination more viable.
[19][20]
The Shevchenko BN350, a nuclear-heated desalination unit
The current trend in dual-purpose facilities is hybrid
configurations, in which the permeate from reverse osmosis desalination
is mixed with distillate from thermal desalination. Basically, two or
more desalination processes are combined along with power production.
Such facilities have been implemented in Saudi Arabia at
Jeddah and
Yanbu.
[21]
A typical
Supercarrier in the US military uses nuclear power to desalinate 400,000 US gallons (1,500,000 l; 330,000 imp gal) of water per day.
[22]
Economics
Costs of desalinating sea water (infrastructure, energy, and maintenance) are generally higher than fresh water from rivers or
groundwater,
water recycling, and
water conservation,
but alternatives are not always available. Desalination costs in 2013
ranged from US$0.45 to $1.00/cubic metre ($US2 to 4/kgal). (1 cubic
meter is about 264 gallons.) More than half of the cost comes directly
from energy cost, and since energy prices are very volatile, actual
costs can vary substantially.
[23]
The cost of untreated fresh water in the developing world can reach US$5/cubic metre.
[24]
Average water consumption and cost of supply by sea water desalination at US$1 per cubic metre(±50%)
| Area |
Consumption USgal/person/day |
Consumption litre/person/day |
Desalinated Water Cost US$/person/day |
| USA |
100 |
378 |
0.38 |
| Europe |
50 |
189 |
0.19 |
| Africa |
15 |
57 |
0.06 |
| UN recommended minimum |
13 |
49 |
0.05 |
Factors that determine the costs for desalination include capacity
and type of facility, location, feed water, labor, energy, financing and
concentrate disposal. Desalination
stills control pressure, temperature and brine concentrations to optimize efficiency.
Nuclear-powered desalination might be economical on a large scale.
[25][26]
While noting costs are falling, and generally positive about the
technology for affluent areas in proximity to oceans, a 2004 study
argued, "Desalinated water may be a solution for some water-stress
regions, but not for places that are poor, deep in the interior of a
continent, or at high elevation. Unfortunately, that includes some of
the places with biggest water problems.", and, "Indeed, one needs to
lift the water by 2,000 m (6,600 ft), or transport it over more than
1,600 km (990 mi) to get transport costs equal to the desalination
costs. Thus, it may be more economical to transport fresh water from
somewhere else than to desalinate it. In places far from the sea, like
New Delhi, or in high places, like
Mexico City,
transport costs could match desalination costs. Desalinated water is
also expensive in places that are both somewhat far from the sea and
somewhat high, such as
Riyadh and
Harare. By contrast in other locations transport costs are much less, such as
Beijing,
Bangkok,
Zaragoza,
Phoenix, and, of course, coastal cities like
Tripoli."
[27] After desalination at
Jubail, Saudi Arabia, water is pumped 200 mi (320 km) inland to
Riyadh.
[28] For coastal cities, desalination is increasingly viewed as a competitive choice.
In 2014, the Israeli facilities of Hadera, Palmahim, Ashkelon, and
Sorek were desalinizing water for less than US$0.40 per cubic meter.
[29] As of 2006, Singapore was desalinating water for US$0.49 per cubic meter.
[30] The city of
Perth began operating a reverse osmosis seawater desalination plant in 2006.
[31] A desalination plant now operates in
Sydney,
[32] and the
Wonthaggi desalination plant was under construction in
Wonthaggi, Victoria.
The Perth desalination plant is powered partially by renewable energy from the
Emu Downs Wind Farm.
[33][34] A wind farm at
Bungendore in
New South Wales was purpose-built to generate enough
renewable energy to offset the Sydney plant's energy use,
[35] mitigating concerns about harmful
greenhouse gas emissions.
In December 2007, the South Australian government announced it would
build a seawater desalination plant for the city of Adelaide, Australia,
located at
Port Stanvac. The desalination plant was to be funded by raising water rates to achieve full cost recovery.
[36][37]
A January 17, 2008, article in the
Wall Street Journal stated, "In November, Connecticut-based Poseidon Resources Corp. won a key regulatory approval to build the $300 million water-
desalination plant in
Carlsbad, north of
San Diego.
The facility would produce 50,000,000 US gallons (190,000,000 l;
42,000,000 imp gal) of drinking water per day, enough to supply about
100,000 homes.
[38] As of June 2012, the cost for the desalinated water had risen to $2,329 per acre-foot.
[39] Each $1,000 per acre-foot works out to $3.06 for 1,000 gallons, or $.81 per cubic meter.
[40]
Poseidon Resources made an unsuccessful attempt to construct a
desalination plant in Tampa Bay, FL, in 2001. The board of directors of
Tampa Bay Water
was forced to buy the plant from Poseidon in 2001 to prevent a third
failure of the project. Tampa Bay Water faced five years of engineering
problems and operation at 20% capacity to protect marine life. The
facility reached capacity only in 2007.
[41]
In 2008, a
Energy Recovery Inc. was desalinating water for $0.46 per cubic meter.
[42]
Environmental
Intake
In the
United States, cooling water intake structures are regulated by the
Environmental Protection Agency
(EPA). These structures can have the same impacts to the environment as
desalination facility intakes. According to EPA, water intake
structures cause adverse environmental impact by sucking fish and
shellfish or their eggs into an industrial system. There, the organisms
may be killed or injured by heat, physical stress, or chemicals. Larger
organisms may be killed or injured when they become trapped against
screens at the front of an intake structure.
[43] Alternative intake types that mitigagte these impacts include beach wells, but they require more energy and higher costs.
[44]
The
Kwinana Desalination Plant opened in Perth in 2007. Water there and at Queensland's
Gold Coast Desalination Plant and Sydney's
Kurnell Desalination Plant is withdrawn at 0.1 m/s (0.33 ft/s), which is slow enough to let fish escape. The plant provides nearly 140,000 m
3 (4,900,000 cu ft) of clean water per day.
[33]
Outflow
Desalination processes produce large quantities of
brine,
possibly at above ambient temperature, and contain residues of
pretreatment and cleaning chemicals, their reaction byproducts and heavy
metals due to corrosion.
[45]
Chemical pretreatment and cleaning are a necessity in most desalination
plants, which typically includes prevention of biofouling, scaling,
foaming and corrosion in thermal plants, and of biofouling, suspended
solids and scale deposits in membrane plants.
[46]
To limit the environmental impact of returning the brine to the
ocean, it can be diluted with another stream of water entering the
ocean, such as the outfall of a
wastewater treatment
or power plant. With medium to large power plant and desalination
plants, the power plant's cooling water flow is likely to be several
times larger than that of the desalination plant, reducing the salinity
of the combination. Another method to reduce the dilute the brine is to
mix it via a diffuser in a mixing zone. For example, once a pipeline
containing the brine reaches the sea floor, it can split into many
branches, each releasing brine gradually through small holes along its
length. Mixing can be combined with power plant or wastewater plant
dilution.
Brine is denser than seawater and therefore sinks to the ocean bottom
and can damage the ecosystem. Careful reintroduction can minimize this
problem. Typical ocean conditions allow for rapid dilution, thereby
minimizing harm.
Alternatives without pollution
Some methods of desalination, particularly in combination with
evaporation ponds,
solar stills, and
condensation trap (
solar desalination),
do not discharge brine. They do not use chemicals or burn fossil fuels.
They do not work with membranes or other critical parts, such as
components that include heavy metals, thus do not produce toxic waste
(and high maintenance).
A new approach that works like a solar still, but on the scale of industrial evaporation ponds is the
integrated biotectural system.
[47]
It can be considered "full desalination" because it converts the entire
amount of saltwater intake into distilled water. One of the advantages
of this system is the feasibility for inland operation. Standard
advantages also include no air pollution and no temperature increase of
endangered natural water bodies from power plant cooling-water
discharge. Another important advantage is the production of sea salt for
industrial and other uses. As of 2015, 50% of the world's sea salt
production relies on fossil energy sources.
[48]
Alternatives to desalination
Increased
water conservation
and efficiency remain the most cost-effective approaches in areas with a
large potential to improve the efficiency of water use practices.
[49] Wastewater reclamation provides multiple benefits over desalination.
[50] Urban runoff and storm water capture also provide benefits in treating, restoring and recharging groundwater.
[51]
A proposed alternative to desalination in the American Southwest is
the commercial importation of bulk water from water-rich areas either by
oil tankers
converted to water carriers, or pipelines. The idea is politically
unpopular in Canada, where governments imposed trade barriers to bulk
water exports as a result of a
North American Free Trade Agreement (NAFTA) claim.
[52]
Public health concerns
Desalination
removes iodine from water and could increase the risk of iodine
deficiency disorders. Israeli researchers claimed a possible link
between seawater desalination and iodine deficiency,
[53] finding deficits among
euthyroid adults exposed to iodine-poor water
[54] concurrently with an increasing proportion of their area's drinking water from seawater reverse osmosis (SWRO).
[55] They later found probable iodine deficiency disorders in a population reliant on desalinated seawater.
[56]
Experimental techniques
Other desalination techniques include:
Waste heat
Diesel generators
commonly provide electricity in remote areas. About 40%–50% of the
energy output is low-grade heat that leaves the engine via the exhaust.
Connecting a
membrane distillation system to the diesel engine exhaust repurposes this low-grade heat for desalination. The system actively cools the
diesel generator,
improving its efficiency and increasing its electricity output. This
results in an energy-neutral desalination solution. An example plant was
commissioned by Dutch company
Aquaver in March 2014 for
Gulhi,
Maldives.
[57][58]
Low-temperature thermal
Originally stemming from
ocean thermal energy conversion research,
low-temperature thermal desalination (LTTD) takes advantage of water boiling at low pressure, even at
ambient temperature.
The system uses pumps to create a low-pressure, low-temperature
environment in which water boils at a temperature gradient of 8–10 °C
(46–50 °F) between two volumes of water. Cool ocean water is supplied
from depths of up to 600 m (2,000 ft). This water is pumped through
coils to condense the water vapor. The resulting condensate is purified
water. LTTD may take advantage of the temperature gradient available at
power plants, where large quantities of warm wastewater are discharged
from the plant, reducing the energy input needed to create a temperature
gradient.
[59]
Experiments were conducted in the US and Japan to test the approach.
In Japan, a spray-flash evaporation system was tested by Saga University.
[60]
In Hawaii, the National Energy Laboratory tested an open-cycle OTEC
plant with fresh water and power production using a temperature
difference of 20 C° between surface water and water at a depth of around
500 m (1,600 ft). LTTD was studied by India's National Institute of
Ocean Technology (NIOT) in 2004. Their first LTTD plant opened in 2005
at Kavaratti in the
Lakshadweep
islands. The plant's capacity is 100,000 L (22,000 imp gal;
26,000 US gal)/day, at a capital cost of INR 50 million (€922,000). The
plant uses deep water at a temperature of 10 to 12 °C (50 to 54 °F).
[61] In 2007, NIOT opened an experimental, floating LTTD plant off the coast of
Chennai,
with a capacity of 1,000,000 L (220,000 imp gal; 260,000 US gal)/day. A
smaller plant was established in 2009 at the North Chennai Thermal
Power Station to prove the LTTD application where power plant cooling
water is available.
[59][62][63]
Thermoionic process
In October 2009, Saltworks Technologies announced a process that uses solar or other thermal heat to drive an
ionic current that removes all
sodium and
chlorine ions from the water using ion-exchange membranes.
[64]
Evaporation and condensation for crops
The
Seawater greenhouse uses natural evaporation and condensation processes inside a
greenhouse powered by solar energy to grow crops in arid coastal land.
Other approaches
Forward osmosis
One process was commercialized by Modern Water PLC using
forward osmosis, with a number of plants reported to be in operation.
[65][66][67]
Small-scale solar
The United States, France and the United Arab Emirates are working to develop practical
solar desalination.
[68]
AquaDania's WaterStillar has been installed at Dahab, Egypt, and in
Playa del Carmen, Mexico. In this approach, a solar thermal collector
measuring two square metres can distill from 40 to 60 litres per day
from any local water source – five times more than conventional stills.
It eliminates the need for plastic
PET bottles or energy-consuming water transport.
[69]
In Central California, a startup company WaterFX is developing a
solar-powered method of desalination that can enable the use of local
water, including runoff water that can be treated and used again. Salty
groundwater in the region would be treated to become freshwater, and in
areas near the ocean, seawater could be treated.
[70]
Passarell
The
Passarell process uses reduced atmospheric pressure rather than heat to
drive evaporative desalination. The pure water vapor generated by
distillation is then compressed and condensed using an advanced
compressor. The compression process improves distillation efficiency by
creating the reduced pressure in the evaporation chamber. The compressor
centrifuges
the pure water vapor after it is drawn through a demister (removing
residual impurities) causing it to compress against tubes in the
collection chamber. The compression of the vapor increases its
temperature. The heat is transferred to the input water falling in the
tubes, vaporizing the water in the tubes. Water vapor condenses on the
outside of the tubes as product water. By combining several physical
processes, Passarell enables most of the system's energy to be recycled
through its evaporation, demisting, vapor compression, condensation, and
water movement processes.
[71]
Geothermal
Geothermal energy can drive desalination. In most locations,
geothermal desalination beats using scarce groundwater or surface water, environmentally and economically.
[citation needed]
Nanotechnology
Nanotube membranes
may prove to be effective for water filtration and desalination
processes that would require substantially less energy than reverse
osmosis.
[72]
Hermetic, sulphonated nano-composite membranes have shown to be
capable of reducing almost all forms of contamination to the parts per
billion level. These nano-materials, using a non-reverse osmosis
process, have little or no susceptibility to high salt concentration
levels.
[73][74][75]
Abstracted animation of the nanoscale graphene membrane desalination process.
Biomimesis
Biomimetic membranes are another approach.
[76]
Electrochemical
In
2008, Siemens Water Technologies announced technology that applied
electric fields to desalinate one cubic meter of water while using only a
purported 1.5 kWh of energy. If accurate, this process would consume
one-half the energy of other processes.
[77] As of 2012 a demonstration plant was operating in Singapore.
[78]
Researchers at the University of Texas at Austin and the University of
Marburg are developing more efficient methods of electrochemically
mediated seawater desalination.
[79]
Freeze-thaw
Freeze-thaw desalination uses freezing to remove fresh water from frozen seawater.
[80]
Electrokinetic shocks
Membraneless desalination at ambient temperature and pressure used electrokinetic shocks waves.
[81]
Anions and cations in salt water are exchanged for carbonate anions and
calcium cations respectively using electrokinetic shockwaves. Calcium
and carbonate ions react to form
calcium carbonate, which precipitates, leaving fresh water. Theoretical energy efficiency of this method is on par with
electrodialysis and
reverse osmosis.
Facilities
Estimates vary widely between 15,000–20,000 desalination plants producing more than 20,000 m
3/day. Micro desalination plants operate near almost every
natural gas or
fracking facility is found in the United States.
[citation needed]
In nature
Mangrove leaf with salt crystals
Evaporation of water over the oceans in the
water cycle is a natural desalination process.
The formation of
sea ice produces ice with little salt, much lower than in seawater.
Seabirds distill seawater using
countercurrent exchange in a
gland with a
rete mirabile. The gland
secretes highly concentrated brine
stored near the nostrils above the beak. The bird then "sneezes" the
brine out. As freshwater is not usually available in their environments,
some seabirds, such as
pelicans,
petrels,
albatrosses,
gulls and
terns, possess this gland, which allows them to drink the salty water from their environments while they are far from land.
[82][83]
Mangrove
trees grow in seawater; they secrete salt by trapping it in parts of
the root, which are then eaten by animals (usually crabs). Additional
salt is removed by storing it in leaves that fall off. Some types of
mangroves have glands on their leaves, which work in a similar way to
the seabird desalination gland. Salt is extracted to the leaf exterior
as small
crystals, which then fall off the leaf.
Willow trees and
reeds absorb salt and other contaminants, effectively desalinating the water. This is used in artificial
constructed wetlands, for treating
sewage.
[citation needed]
See also
References




No comments:
Post a Comment