Photosynthesis
From Wikipedia, the free encyclopedia
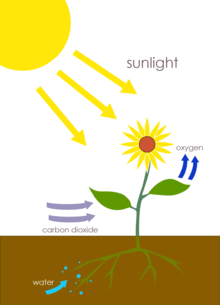
Schematic of photosynthesis in plants. The carbohydrates produced are stored in or used by the plant.
Overall equation for the type of photosynthesis that occurs in plants
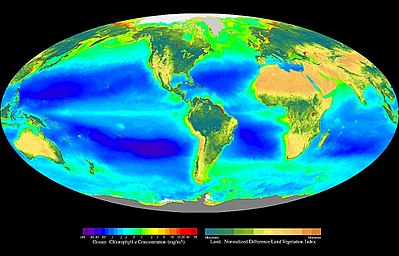
Composite image showing the global distribution of photosynthesis, including both oceanic
phytoplankton and terrestrial
vegetation. Dark red and blue-green indicate regions of high photosynthetic activity in ocean and land respectively.
Photosynthesis is a process used by plants and other organisms to convert
light energy, normally from the
Sun, into
chemical energy that can be later
released to fuel the organisms' activities (
energy transformation). This chemical energy is stored in
carbohydrate molecules, such as
sugars, which are synthesized from
carbon dioxide and
water – hence the name photosynthesis, from the
Greek φῶς,
phōs, "light", and
σύνθεσις,
synthesis, "putting together".
[1][2][3] In most cases, oxygen is also released as a waste product. Most
plants, most
algae, and
cyanobacteria perform photosynthesis; such organisms are called
photoautotrophs. Photosynthesis maintains
atmospheric oxygen levels and supplies all of the organic compounds and most of the energy necessary for
life on Earth.
[4]
Although photosynthesis is performed differently by different
species, the process always begins when energy from light is absorbed by
proteins called
reaction centres that contain green
chlorophyll pigments. In plants, these proteins are held inside
organelles called
chloroplasts, which are most abundant in leaf cells, while in bacteria they are embedded in the
plasma membrane. In these light-dependent reactions, some energy is used to strip
electrons
from suitable substances, such as water, producing oxygen gas. The
hydrogen freed by water splitting is used in the creation of two further
compounds: reduced
nicotinamide adenine dinucleotide phosphate (NADPH) and
adenosine triphosphate (ATP), the "energy currency" of cells.
In plants, algae and cyanobacteria, sugars are produced by a subsequent sequence of light-independent reactions called the
Calvin cycle, but some bacteria use different mechanisms, such as the
reverse Krebs cycle. In the Calvin cycle, atmospheric carbon dioxide is
incorporated into already existing organic carbon compounds, such as
ribulose bisphosphate (RuBP).
[5] Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then
reduced and removed to form further carbohydrates, such as
glucose.
The first photosynthetic organisms probably
evolved early in the
evolutionary history of life and most likely used
reducing agents such as
hydrogen or
hydrogen sulfide, rather than water, as sources of electrons.
[6] Cyanobacteria appeared later; the excess oxygen they produced contributed to the
oxygen catastrophe,
[7] which rendered the
evolution of complex life possible. Today, the average rate of energy capture by photosynthesis globally is approximately 130
terawatts,
[8][9][10] which is about three times the current
power consumption of human civilization.
[11] Photosynthetic organisms also convert around 100–115 thousand million metric tonnes of carbon into
biomass per year.
[12][13]
Overview
Photosynthesis changes sunlight into chemical energy, splits water to liberate O2, and fixes CO2 into sugar.
Photosynthetic organisms are
photoautotrophs, which means that they are able to
synthesize
food directly from carbon dioxide and water using energy from light.
However, not all organisms that use light as a source of energy carry
out photosynthesis, since
photoheterotrophs use organic compounds, rather than carbon dioxide, as a source of carbon.
[4] In plants, algae and cyanobacteria, photosynthesis releases oxygen. This is called
oxygenic photosynthesis. Although there are some differences between oxygenic photosynthesis in
plants,
algae, and
cyanobacteria, the overall process is quite similar in these organisms. However, there are some types of bacteria that carry out
anoxygenic photosynthesis. These consume carbon dioxide but do not release oxygen.
Carbon dioxide is converted into sugars in a process called
carbon fixation. Carbon fixation is an
endothermic redox
reaction, so photosynthesis needs to supply both a source of energy to
drive this process, and the electrons needed to convert carbon dioxide
into a
carbohydrate via a reduction reaction. The addition of electrons to a chemical species is called a
reduction reaction. In general outline and in effect, photosynthesis is the opposite of
cellular respiration, in which glucose and other compounds are oxidized to produce carbon dioxide and water, and to release chemical energy (an
exothermic reaction) to drive the organism's
metabolism.
The two processes, of reduction of carbon dioxide to carbohydrate and
then the later oxidation of the carbohydrate, take place through a
different sequence of chemical reactions and in different cellular
compartments.
The general
equation for photosynthesis as first proposed by
Cornelius van Niel is therefore:
[14]
- CO2 + 2H2A + photons → [CH2O] + 2A + H2O
- carbon dioxide + electron donor + light energy → carbohydrate + oxidized electron donor + water
Since water is used as the electron donor in oxygenic photosynthesis, the equation for this process is:
- CO2 + 2H2O + photons → [CH2O] + O2 + H2O
- carbon dioxide + water + light energy → carbohydrate + oxygen + water
This equation emphasizes that water is both a reactant in the
light-dependent reaction and a product of the
light-independent reaction, but canceling
n water molecules from each side gives the net equation:
- CO2 + H2O + photons → [CH2O] + O2
- carbon dioxide + water + light energy → carbohydrate + oxygen
Other processes substitute other compounds (such as
arsenite) for water in the electron-supply role; for example some microbes use sunlight to oxidize arsenite to
arsenate:
[15] The equation for this reaction is:
- CO2 + (AsO33−) + photons → (AsO43−) + CO[16]
- carbon dioxide + arsenite + light energy → arsenate + carbon monoxide (used to build other compounds in subsequent reactions)
Photosynthesis occurs in two stages. In the first stage,
light-dependent reactions or
light reactions capture the energy of light and use it to make the energy-storage molecules
ATP and
NADPH. During the second stage, the
light-independent reactions use these products to capture and reduce carbon dioxide.
Most organisms that utilize photosynthesis to produce oxygen use
visible light to do so, although at least three use shortwave
infrared or, more specifically, far-red radiation.
[17]
Archaeobacteria use a simpler method using a pigment similar to the pigments used for vision. The
archaearhodopsin
changes its configuration in response to sunlight, acting as a proton
pump. This produces a proton gradient more directly which is then
converted to chemical energy. The process does not involve carbon
dioxide fixation and does not release oxygen. It seems to have evolved
separately.
[18][19]
Photosynthetic membranes and organelles
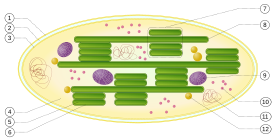
Chloroplast ultrastructure:
1. outer membrane
2. intermembrane space
3. inner membrane (1+2+3: envelope)
4. stroma (aqueous fluid)
5. thylakoid lumen (inside of thylakoid)
6. thylakoid membrane
7. granum (stack of thylakoids)
8. thylakoid (lamella)
9. starch
10. ribosome
11. plastidial DNA
12. plastoglobule (drop of lipids)
Main articles:
Chloroplast and
Thylakoid
In photosynthetic bacteria, the proteins that gather light for photosynthesis are embedded in
cell membranes. In its simplest form, this involves the membrane surrounding the cell itself.
[20] However, the membrane may be tightly folded into cylindrical sheets called
thylakoids,
[21] or bunched up into round
vesicles called
intracytoplasmic membranes.
[22]
These structures can fill most of the interior of a cell, giving the
membrane a very large surface area and therefore increasing the amount
of light that the bacteria can absorb.
[21]
In plants and algae, photosynthesis takes place in
organelles called
chloroplasts. A typical
plant cell
contains about 10 to 100 chloroplasts. The chloroplast is enclosed by a
membrane. This membrane is composed of a phospholipid inner membrane, a
phospholipid outer membrane, and an intermembrane space between them.
Enclosed by the membrane is an aqueous fluid called the stroma. Embedded
within the stroma are stacks of thylakoids (grana), which are the site
of photosynthesis. The thylakoids appear as flattened disks. The
thylakoid itself is enclosed by the thylakoid membrane, and within the
enclosed volume is a lumen or thylakoid space. Embedded in the thylakoid
membrane are integral and
peripheral membrane protein complexes of the photosynthetic system, including the pigments that absorb light energy.
Plants absorb light primarily using the
pigment chlorophyll.
The green part of the light spectrum is not absorbed but is reflected
which is the reason that most plants have a green color. Besides
chlorophyll, plants also use pigments such as
carotenes and
xanthophylls.
[23] Algae also use chlorophyll, but various other pigments are present, such as
phycocyanin,
carotenes, and
xanthophylls in
green algae,
phycoerythrin in
red algae (rhodophytes) and
fucoxanthin in
brown algae and
diatoms resulting in a wide variety of colors.
These pigments are embedded in plants and algae in complexes called
antenna proteins. In such proteins, the pigments are arranged to work
together. Such a combination of proteins is also called a
light-harvesting complex.
Although all cells in the green parts of a plant have chloroplasts,
the majority of those are found in specially adapted structures called
leaves. Certain species adapted to conditions of strong sunlight and
aridity, such as many
Euphorbia and
cactus species, have their main photosynthetic organs in their stems. The cells in the interior tissues of a leaf, called the
mesophyll,
can contain between 450,000 and 800,000 chloroplasts for every square
millimeter of leaf. The surface of the leaf is coated with a
water-resistant
waxy cuticle that protects the leaf from excessive
evaporation of water and decreases the absorption of
ultraviolet or
blue light to reduce
heating. The transparent
epidermis layer allows light to pass through to the
palisade mesophyll cells where most of the photosynthesis takes place.
Light-dependent reactions
Light-dependent reactions of photosynthesis at the thylakoid membrane
In the
light-dependent reactions, one molecule of the
pigment chlorophyll absorbs one
photon and loses one
electron. This electron is passed to a modified form of chlorophyll called
pheophytin, which passes the electron to a
quinone molecule, starting the flow of electrons down an
electron transport chain that leads to the ultimate reduction of
NADP to
NADPH. In addition, this creates a
proton gradient (energy gradient) across the
chloroplast membrane, which is used by
ATP synthase in the synthesis of
ATP. The chlorophyll molecule ultimately regains the electron it lost when a water molecule is split in a process called
photolysis, which releases a
dioxygen (O
2) molecule as a waste product.
The overall equation for the light-dependent reactions under the conditions of non-cyclic electron flow in green plants is:
[24]
- 2 H2O + 2 NADP+ + 3 ADP + 3 Pi + light → 2 NADPH + 2 H+ + 3 ATP + O2
Not all
wavelengths of light can support photosynthesis. The photosynthetic action spectrum depends on the type of
accessory pigments present. For example, in green plants, the
action spectrum resembles the
absorption spectrum for
chlorophylls and
carotenoids
with peaks for violet-blue and red light. In red algae, the action
spectrum is blue-green light, which allows these algae to use the blue
end of the spectrum to grow in the deeper waters that filter out the
longer wavelengths (red light) used by above ground green plants. The
non-absorbed part of the
light spectrum
is what gives photosynthetic organisms their color (e.g., green plants,
red algae, purple bacteria) and is the least effective for
photosynthesis in the respective organisms.
Z scheme
In plants,
light-dependent reactions occur in the
thylakoid membranes of the
chloroplasts where they drive the synthesis of ATP and NADPH. The light-dependent reactions are of two forms: cyclic and non-cyclic.
In the non-cyclic reaction, the
photons are captured in the light-harvesting
antenna complexes of
photosystem II by
chlorophyll and other
accessory pigments (see diagram at right). The absorption of a photon by the antenna complex frees an electron by a process called
photoinduced charge separation.
The antenna system is at the core of the chlorophyll molecule of the
photosystem II reaction center. That freed electron is transferred to
the primary electron-acceptor molecule, pheophytin. As the electrons are
shuttled through an
electron transport chain (the so-called
Z-scheme shown in the diagram), it initially functions to generate a
chemiosmotic potential by pumping proton cations (H
+) across the membrane and into the thylakoid space. An
ATP synthase enzyme uses that chemiosmotic potential to make ATP during photophosphorylation, whereas
NADPH is a product of the terminal
redox reaction in the
Z-scheme. The electron enters a chlorophyll molecule in
Photosystem I. There it is further excited by the light absorbed by that
photosystem. The electron is then passed along a chain of
electron acceptors
to which it transfers some of its energy. The energy delivered to the
electron acceptors is used to move hydrogen ions across the thylakoid
membrane into the lumen. The electron is eventually used to reduce the
co-enzyme NADP with a H
+ to NADPH (which has functions in the light-independent reaction); at that point, the path of that electron ends.
The cyclic reaction is similar to that of the non-cyclic, but differs
in that it generates only ATP, and no reduced NADP (NADPH) is created.
The cyclic reaction takes place only at photosystem I. Once the electron
is displaced from the photosystem, the electron is passed down the
electron acceptor molecules and returns to photosystem I, from where it
was emitted, hence the name
cyclic reaction.
Water photolysis
The NADPH is the main
reducing agent
produced by chloroplasts, which then goes on to provide a source of
energetic electrons in other cellular reactions. Its production leaves
chlorophyll in photosystem I with a deficit of electrons (chlorophyll
has been oxidized), which must be balanced by some other reducing agent
that will supply the missing electron. The excited electrons lost from
chlorophyll from
photosystem I are supplied from the electron transport chain by
plastocyanin. However, since
photosystem II is the first step of the
Z-scheme, an external source of electrons is required to reduce its oxidized
chlorophyll a
molecules. The source of electrons in green-plant and cyanobacterial
photosynthesis is water. Two water molecules are oxidized by four
successive charge-separation reactions by photosystem II to yield a
molecule of diatomic
oxygen and four
hydrogen ions; the electrons yielded are transferred to a redox-active
tyrosine residue that then reduces the oxidized chlorophyll
a
(called P680) that serves as the primary light-driven electron donor in
the photosystem II reaction center. That photo receptor is in effect
reset and is then able to repeat the absorption of another photon and
the release of another photo-dissociated electron. The oxidation of
water is
catalyzed in photosystem II by a redox-active structure that contains four
manganese ions and a calcium ion; this
oxygen-evolving complex
binds two water molecules and contains the four oxidizing equivalents
that are used to drive the water-oxidizing reaction. Photosystem II is
the only known biological
enzyme
that carries out this oxidation of water. The hydrogen ions released
contribute to the transmembrane chemiosmotic potential that leads to ATP
synthesis. Oxygen is a waste product of light-dependent reactions, but
the majority of organisms on Earth use oxygen for
cellular respiration, including photosynthetic organisms.
[25][26]
Light-independent reactions
Calvin cycle
In the
light-independent (or "dark") reactions, the
enzyme RuBisCO captures
CO2 from the
atmosphere and, in a process called the
Calvin-Benson cycle,
it uses the newly formed NADPH and releases three-carbon sugars, which
are later combined to form sucrose and starch. The overall equation for
the light-independent reactions in green plants is
[24]:128
- 3 CO2 + 9 ATP + 6 NADPH + 6 H+ → C3H6O3-phosphate + 9 ADP + 8 Pi + 6 NADP+ + 3 H2O
Overview of the Calvin cycle and carbon fixation
Carbon fixation produces the intermediate three-carbon sugar product,
which is then converted to the final carbohydrate products. The simple
carbon sugars produced by photosynthesis are then used in the forming of
other organic compounds, such as the building material
cellulose, the precursors for
lipid and
amino acid biosynthesis, or as a fuel in
cellular respiration. The latter occurs not only in plants but also in
animals when the energy from plants is passed through a
food chain.
The fixation or reduction of carbon dioxide is a process in which
carbon dioxide combines with a five-carbon sugar,
ribulose 1,5-bisphosphate, to yield two molecules of a three-carbon compound,
glycerate 3-phosphate, also known as 3-phosphoglycerate. Glycerate 3-phosphate, in the presence of
ATP and
NADPH produced during the light-dependent stages, is reduced to
glyceraldehyde 3-phosphate. This product is also referred to as 3-phosphoglyceraldehyde (
PGAL) or, more generically, as
triose
phosphate. Most (5 out of 6 molecules) of the glyceraldehyde
3-phosphate produced is used to regenerate ribulose 1,5-bisphosphate so
the process can continue. The triose phosphates not thus "recycled"
often condense to form
hexose phosphates, which ultimately yield
sucrose,
starch and
cellulose. The sugars produced during carbon
metabolism yield carbon skeletons that can be used for other metabolic reactions like the production of
amino acids and
lipids.
Carbon concentrating mechanisms
On land
In hot and dry conditions, plants close their
stomata to prevent water loss. Under these conditions, CO
2 will decrease and oxygen gas, produced by the light reactions of photosynthesis, will increase, causing an increase of
photorespiration by the
oxygenase activity of
ribulose-1,5-bisphosphate carboxylase/oxygenase and decrease in carbon fixation. Some plants have
evolved mechanisms to increase the CO
2 concentration in the leaves under these conditions.
[27]
Plants that use the
C4 carbon fixation process chemically fix carbon dioxide in the cells of the mesophyll by adding it to the three-carbon molecule
phosphoenolpyruvate (PEP), a reaction catalyzed by an enzyme called
PEP carboxylase, creating the four-carbon organic acid
oxaloacetic acid. Oxaloacetic acid or
malate synthesized by this process is then translocated to specialized
bundle sheath cells where the enzyme
RuBisCO and other Calvin cycle enzymes are located, and where CO
2 released by
decarboxylation of the four-carbon acids is then fixed by RuBisCO activity to the three-carbon
3-phosphoglyceric acids. The physical separation of RuBisCO from the oxygen-generating light reactions reduces photorespiration and increases CO
2 fixation and, thus, the
photosynthetic capacity of the leaf.
[28] C
4 plants can produce more sugar than C
3 plants in conditions of high light and temperature. Many important crop plants are C
4 plants, including maize, sorghum, sugarcane, and millet. Plants that do not use PEP-carboxylase in carbon fixation are called
C3 plants
because the primary carboxylation reaction, catalyzed by RuBisCO,
produces the three-carbon 3-phosphoglyceric acids directly in the
Calvin-Benson cycle. Over 90% of plants use C
3 carbon fixation, compared to 3% that use C
4 carbon fixation;
[29] however, the evolution of C
4 in over 60 plant lineages makes it a striking example of
convergent evolution.
[27]
Xerophytes, such as
cacti and most
succulents, also use PEP carboxylase to capture carbon dioxide in a process called
Crassulacean acid metabolism (CAM). In contrast to C
4 metabolism, which
physically separates the CO
2 fixation to PEP from the Calvin cycle, CAM
temporally separates these two processes. CAM plants have a different leaf anatomy from C
3 plants, and fix the CO
2 at night, when their stomata are open. CAM plants store the CO
2 mostly in the form of
malic acid via carboxylation of
phosphoenolpyruvate to oxaloacetate, which is then reduced to malate. Decarboxylation of malate during the day releases CO
2 inside the leaves, thus allowing carbon fixation to 3-phosphoglycerate by RuBisCO. Sixteen thousand species of plants use CAM.
[30]
In water
Cyanobacteria possess
carboxysomes, which increase the concentration of CO
2 around RuBisCO to increase the rate of photosynthesis. An enzyme,
carbonic anhydrase, located within the carboxysome releases CO
2 from the dissolved hydrocarbonate ions (HCO
3−). Before the CO
2 diffuses out it is quickly sponged up by RuBisCO, which is concentrated within the carboxysomes. HCO
3− ions are made from CO
2
outside the cell by another carbonic anhydrase and are actively pumped
into the cell by a membrane protein. They cannot cross the membrane as
they are charged, and within the cytosol they turn back into CO
2 very slowly without the help of carbonic anhydrase. This causes the HCO
3− ions to accumulate within the cell from where they diffuse into the carboxysomes.
[31] Pyrenoids in
algae and
hornworts also act to concentrate CO
2 around rubisco.
[32]
Order and kinetics
The overall process of photosynthesis takes place in four stages:
[13]
Efficiency
Probability distribution resulting from one-dimensional discrete time
random walks. The quantum walk created using the Hadamard coin is
plotted (blue) vs a classical walk (red) after 50 time steps.
Plants usually convert light into
chemical energy with a
photosynthetic efficiency of 3–6%.
[33] Absorbed light that is unconverted is dissipated primarily as heat, with a small fraction (1–2%)
[34] re-emitted as
chlorophyll fluorescence at longer (redder) wavelengths.
Actual plants' photosynthetic efficiency varies with the frequency of
the light being converted, light intensity, temperature and proportion
of carbon dioxide in the atmosphere, and can vary from 0.1% to 8%.
[35] By comparison,
solar panels convert light into
electric energy at an efficiency of approximately 6–20% for mass-produced panels, and above 40% in laboratory devices.
Photosynthesis measurement systems are not designed to directly
measure the amount of light absorbed by the leaf. But analysis of
chlorophyll-fluorescence, P700- and P515-absorbance and gas exchange
measurements reveal detailed information about e.g. the photosystems,
quantum efficiency and the CO
2 assimilation rates. With some instruments even wavelength-dependency of the photosynthetic efficiency can be analyzed.
[36]
A phenomenon known as
quantum walk
increases the efficiency of the energy transport of light
significantly. In the photosynthetic cell of an algae, bacterium, or
plant, there are light-sensitive molecules called
chromophores
arranged in an antenna-shaped structure named a photocomplex. When a
photon is absorbed by a chromophore, it is converted into a
quasiparticle referred to as an
exciton,
which jumps from chromophore to chromophore towards the reaction center
of the photocomplex, a collection of molecules that traps its energy in
a chemical form that makes it accessible for the cell's metabolism. The
exciton's wave properties enable it to cover a wider area and try out
several possible paths simultaneously, allowing it to instantaneously
"choose" the most efficient route, where it will have the highest
probability of arriving at its destination in the minimum possible time.
Because that quantum walking takes place at temperatures far higher
than quantum phenomena usually occur, it is only possible over very
short distances, due to obstacles in the form of destructive
interference that come into play. These obstacles cause the particle to
lose its wave properties for an instant before it regains them once
again after it is freed from its locked position through a classic
"hop". The movement of the electron towards the photo center is
therefore covered in a series of conventional hops and quantum walks.
[37][38][39]
Evolution
-4500 —
–
-4000 —
–
-3500 —
–
-3000 —
–
-2500 —
–
-2000 —
–
-1500 —
–
-1000 —
–
-500 —
–
0 —
Early photosynthetic systems, such as those in
green and
purple sulfur and
green and
purple nonsulfur bacteria, are thought to have been anoxygenic, and used various other molecules as
electron donors rather than water. Green and purple sulfur bacteria are thought to have used
hydrogen and
sulfur as electron donors. Green nonsulfur bacteria used various
amino and other
organic acids
as an electron donor. Purple nonsulfur bacteria used a variety of
nonspecific organic molecules. The use of these molecules is consistent
with the geological evidence that Earth's early atmosphere was highly
reducing at
that time.
[citation needed]
Fossils of what are thought to be
filamentous photosynthetic organisms have been dated at 3.4 billion years old.
[40][41]
The main source of
oxygen in the
Earth's atmosphere derives from
oxygenic photosynthesis, and its first appearance is sometimes referred to as the
oxygen catastrophe. Geological evidence suggests that oxygenic photosynthesis, such as that in
cyanobacteria, became important during the
Paleoproterozoic
era around 2 billion years ago. Modern photosynthesis in plants and
most photosynthetic prokaryotes is oxygenic. Oxygenic photosynthesis
uses water as an electron donor, which is
oxidized to molecular oxygen (
O
2) in the
photosynthetic reaction center.
Symbiosis and the origin of chloroplasts
Several groups of animals have formed
symbiotic relationships with photosynthetic algae. These are most common in
corals,
sponges and
sea anemones. It is presumed that this is due to the particularly simple
body plans and large surface areas of these animals compared to their volumes.
[42] In addition, a few marine
mollusks Elysia viridis and
Elysia chlorotica
also maintain a symbiotic relationship with chloroplasts they capture
from the algae in their diet and then store in their bodies. This allows
the mollusks to survive solely by photosynthesis for several months at a
time.
[43][44] Some of the genes from the plant
cell nucleus have even been transferred to the slugs, so that the chloroplasts can be supplied with proteins that they need to survive.
[45]
An even closer form of symbiosis may explain the origin of chloroplasts. Chloroplasts have many similarities with
photosynthetic bacteria, including a circular
chromosome, prokaryotic-type
ribosome, and similar proteins in the photosynthetic reaction center.
[46][47] The
endosymbiotic theory suggests that photosynthetic bacteria were acquired (by
endocytosis) by early
eukaryotic cells to form the first
plant cells. Therefore, chloroplasts may be photosynthetic bacteria that adapted to life inside plant cells. Like
mitochondria, chloroplasts possess their own DNA, separate from the
nuclear DNA of their plant host cells and the genes in this chloroplast DNA resemble those found in
cyanobacteria.
[48] DNA in chloroplasts codes for
redox proteins such as those found in the photosynthetic reaction centers. The
CoRR Hypothesis proposes that this
Co-location is required for
Redox
Regulation.
[clarification needed]
Cyanobacteria and the evolution of photosynthesis
The biochemical capacity to use water as the source for electrons in photosynthesis evolved once, in a
common ancestor of extant
cyanobacteria.
The geological record indicates that this transforming event took place
early in Earth's history, at least 2450–2320 million years ago (Ma),
and, it is speculated, much earlier.
[49][50]
Because the Earth's atmosphere contained almost no oxygen during the
estimated development of photosynthesis, it is believed that the first
photosynthetic cyanobacteria did not generate oxygen.
[51] Available evidence from geobiological studies of
Archean (>2500 Ma)
sedimentary rocks
indicates that life existed 3500 Ma, but the question of when oxygenic
photosynthesis evolved is still unanswered. A clear paleontological
window on cyanobacterial
evolution opened about 2000 Ma, revealing an already-diverse biota of blue-green algae.
Cyanobacteria remained the principal
primary producers of oxygen throughout the
Proterozoic Eon (2500–543 Ma), in part because the redox structure of the oceans favored photoautotrophs capable of
nitrogen fixation.
[citation needed] Green algae joined blue-green algae as the major primary producers of oxygen on
continental shelves near the end of the
Proterozoic, but it was only with the
Mesozoic (251–65 Ma) radiations of dinoflagellates, coccolithophorids, and diatoms did the
primary production of oxygen in marine shelf waters take modern form. Cyanobacteria remain critical to
marine ecosystems as primary producers of oxygen in oceanic gyres, as agents of biological nitrogen fixation, and, in modified form, as the
plastids of marine algae.
[52]
The
Oriental hornet (
Vespa orientalis) converts sunlight into electric power using a pigment called
xanthopterin. This is the first evidence of a member of the animal kingdom engaging in photosynthesis.
[53]
Discovery
Although some of the steps in photosynthesis are still not completely
understood, the overall photosynthetic equation has been known since
the 19th century.
Jan van Helmont began the research of the process in the mid-17th century when he carefully measured the
mass
of the soil used by a plant and the mass of the plant as it grew. After
noticing that the soil mass changed very little, he hypothesized that
the mass of the growing plant must come from the water, the only
substance he added to the potted plant. His hypothesis was partially
accurate — much of the gained mass also comes from carbon dioxide as
well as water. However, this was a signaling point to the idea that the
bulk of a plant's
biomass comes from the inputs of photosynthesis, not the soil itself.
Joseph Priestley,
a chemist and minister, discovered that, when he isolated a volume of
air under an inverted jar, and burned a candle in it, the candle would
burn out very quickly, much before it ran out of wax. He further
discovered that a mouse could similarly "injure" air. He then showed
that the air that had been "injured" by the candle and the mouse could
be restored by a plant.
In 1778,
Jan Ingenhousz,
repeated Priestley's experiments. He discovered that it was the
influence of sunlight on the plant that could cause it to revive a mouse
in a matter of hours.
In 1796,
Jean Senebier,
a Swiss pastor, botanist, and naturalist, demonstrated that green
plants consume carbon dioxide and release oxygen under the influence of
light. Soon afterward,
Nicolas-Théodore de Saussure showed that the increase in mass of the plant as it grows could not be due only to uptake of CO
2
but also to the incorporation of water. Thus, the basic reaction by
which photosynthesis is used to produce food (such as glucose) was
outlined.
Cornelis Van Niel made key discoveries explaining the chemistry of photosynthesis. By studying
purple sulfur bacteria and green bacteria he was the first to demonstrate that photosynthesis is a light-dependent
redox reaction, in which hydrogen reduces carbon dioxide.
Robert Emerson discovered two light reactions by testing plant
productivity using different wavelengths of light. With the red alone,
the light reactions were suppressed. When blue and red were combined,
the output was much more substantial. Thus, there were two photosystems,
one absorbing up to 600 nm wavelengths, the other up to 700 nm. The
former is known as PSII, the latter is PSI. PSI contains only
chlorophyll "a", PSII contains primarily chlorophyll "a" with most of
the available chlorophyll "b", among other pigment. These include
phycobilins, which are the red and blue pigments of red and blue algae
respectively, and fucoxanthol for brown algae and diatoms. The process
is most productive when the absorption of quanta are equal in both the
PSII and PSI, assuring that input energy from the antenna complex is
divided between the PSI and PSII system, which in turn powers the
photochemistry.
[13]
Melvin Calvin works in his photosynthesis laboratory.
Robert Hill thought that a complex of reactions consisting of an intermediate to cytochrome b
6
(now a plastoquinone), another is from cytochrome f to a step in the
carbohydrate-generating mechanisms. These are linked by plastoquinone,
which does require energy to reduce cytochrome f for it is a sufficient
reductant. Further experiments to prove that the oxygen developed during
the photosynthesis of green plants came from water, were performed by
Hill in 1937 and 1939. He showed that isolated
chloroplasts give off oxygen in the presence of unnatural reducing agents like
iron oxalate,
ferricyanide or
benzoquinone after exposure to light. The Hill reaction
[54] is as follows:
- 2 H2O + 2 A + (light, chloroplasts) → 2 AH2 + O2
where A is the electron acceptor. Therefore, in light, the electron acceptor is reduced and oxygen is evolved.
Samuel Ruben and
Martin Kamen used
radioactive isotopes to determine that the oxygen liberated in photosynthesis came from the water.
Melvin Calvin and
Andrew Benson, along with
James Bassham,
elucidated the path of carbon assimilation (the photosynthetic carbon
reduction cycle) in plants. The carbon reduction cycle is known as the
Calvin cycle,
which ignores the contribution of Bassham and Benson. Many scientists
refer to the cycle as the Calvin-Benson Cycle, Benson-Calvin, and some
even call it the Calvin-Benson-Bassham (or CBB) Cycle.
Nobel Prize-winning scientist
Rudolph A. Marcus was able to discover the function and significance of the electron transport chain.
Otto Heinrich Warburg and
Dean Burk discovered the I-quantum photosynthesis reaction that splits the CO
2, activated by the respiration.
[55]
Louis N.M. Duysens and
Jan Amesz
discovered that chlorophyll a will absorb one light, oxidize cytochrome
f, chlorophyll a (and other pigments) will absorb another light, but
will reduce this same oxidized cytochrome, stating the two light
reactions are in series.
Development of the concept
In 1893,
Charles Reid Barnes proposed two terms,
photosyntax and
photosynthesis, for the biological process of
synthesis of complex carbon compounds out of carbonic acid, in the presence of chlorophyll, under the influence of light. Over time, the term
photosynthesis
came into common usage as the term of choice. Later discovery of
anoxygenic photosynthetic bacteria and photophosphorylation necessitated
redefinition of the term.
[56]
Factors
The
leaf is the primary site of photosynthesis in plants.
There are three main factors affecting photosynthesis and several corollary factors. The three main are:
[57]
Light intensity (irradiance), wavelength and temperature
Absorbance spectra of free chlorophyll
a (
green) and
b (
red) in a solvent. The
action spectra of chlorophyll molecules are slightly modified
in vivo depending on specific pigment-protein interactions.
The process of photosynthesis provides the main input of free energy
into the biosphere, and is one of four main ways in which radiation is
important for plant life .
[58]
The radiation climate within plant communities is extremely variable, with both time and space.
In the early 20th century,
Frederick Blackman and
Gabrielle Matthaei investigated the effects of light intensity (
irradiance) and temperature on the rate of carbon assimilation.
- At constant temperature, the rate of carbon assimilation varies with
irradiance, increasing as the irradiance increases, but reaching a
plateau at higher irradiance.
- At low irradiance, increasing the temperature has little influence
on the rate of carbon assimilation. At constant high irradiance, the
rate of carbon assimilation increases as the temperature is increased.
These two experiments illustrate several important points: First, it is known that, in general,
photochemical reactions are not affected by
temperature.
However, these experiments clearly show that temperature affects the
rate of carbon assimilation, so there must be two sets of reactions in
the full process of carbon assimilation. These are, of course, the
light-dependent 'photochemical' temperature-independent stage, and the
light-independent, temperature-dependent stage. Second, Blackman's experiments illustrate the concept of
limiting factors.
Another limiting factor is the wavelength of light. Cyanobacteria,
which reside several meters underwater, cannot receive the correct
wavelengths required to cause photoinduced charge separation in
conventional photosynthetic pigments. To combat this problem, a series
of proteins with different pigments surround the reaction center. This
unit is called a
phycobilisome.
[clarification needed]
Carbon dioxide levels and photorespiration
As carbon dioxide concentrations rise, the rate at which sugars are made by the
light-independent reactions increases until limited by other factors.
RuBisCO,
the enzyme that captures carbon dioxide in the light-independent
reactions, has a binding affinity for both carbon dioxide and oxygen.
When the concentration of carbon dioxide is high, RuBisCO will
fix carbon dioxide. However, if the carbon dioxide concentration is low, RuBisCO will bind oxygen instead of carbon dioxide. This process, called
photorespiration, uses energy, but does not produce sugars.
RuBisCO oxygenase activity is disadvantageous to plants for several reasons:
- One product of oxygenase activity is phosphoglycolate (2 carbon) instead of 3-phosphoglycerate
(3 carbon). Phosphoglycolate cannot be metabolized by the Calvin-Benson
cycle and represents carbon lost from the cycle. A high oxygenase
activity, therefore, drains the sugars that are required to recycle
ribulose 5-bisphosphate and for the continuation of the Calvin-Benson cycle.
- Phosphoglycolate is quickly metabolized to glycolate that is toxic
to a plant at a high concentration; it inhibits photosynthesis.
- Salvaging glycolate is an energetically expensive process that uses
the glycolate pathway, and only 75% of the carbon is returned to the
Calvin-Benson cycle as 3-phosphoglycerate. The reactions also produce ammonia (NH3), which is able to diffuse out of the plant, leading to a loss of nitrogen.
-
- A highly simplified summary is:
-
-
- 2 glycolate + ATP → 3-phosphoglycerate + carbon dioxide + ADP + NH3
The salvaging pathway for the products of RuBisCO oxygenase activity is more commonly known as
photorespiration, since it is characterized by light-dependent oxygen consumption and the release of carbon dioxide.
See also
References












No comments:
Post a Comment