A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides. So, when it rains of it splashes up on people's bodies from buses or cars or from their own feet splashing water it's affect on skin and clothes (if it doesn't explode first) would be the stinging of an Acid. Likely, you would want to wear goggles so you didn't get it in your eyes and go blind from this problem and you also wouldn't want to be breathing this in a mist like form because it might harm your lungs. Also, if you take in enough into your body your breathing will not help keep you alive. It is a similar effect to carbon monoxide upon the body. You know, when people go in their garage and close the door and turn the engine on to die. Your body cannot oxygenate and so you eventually die. So, if it doesn't blow up or catch fire you also don't want to breathe it or get it on your skin in any way.
Hydrocyanic Acid is also called:
Isocyanic acid
From Wikipedia, the free encyclopedia
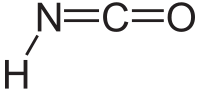 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
Isocyanic acid
|
|
| Identifiers | |
| 75-13-8 420-05-3 (cyanic acid) |
|
| ChEBI | CHEBI:29202 |
| ChemSpider | 6107 |
| Jmol-3D images | Image |
| PubChem | 6347 |
| Properties | |
| HNCO | |
| Molar mass | 43.03 g/mol |
| Appearance | Colorless liquid or gas (b.p. near room temperature) |
| Density | 1.14 g/cm3 (20 °C) |
| Melting point | −86 °C (−123 °F; 187 K)[1] |
| Boiling point | 23.5 °C (74.3 °F; 296.6 K) |
| Dissolves | |
| Solubility | Soluble in benzene, toluene, ether |
| Hazards | |
| Main hazards | Poisonous |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| |
|
| Infobox references | |
Contents
Preparation and reactions
Isocyanic acid can be made by protonation of the cyanate anion, such as from salts like potassium cyanate, by either gaseous hydrogen chloride or acids such as oxalic acid.[3]- H+ + NCO- → HNCO
- C3H3N3O3 → 3 HNCO
- HNCO + H2O → CO2 + NH3
Isocyanic acid reacts with amines to give ureas (carbamides):
- HNCO + RNH2 → RNHC(O)NH2.
HNCO adds across electron-rich double bonds, such as vinylethers, to give the corresponding isocyanates.
Isocyanic acid is also present in various forms of smoke, including smog and cigarette smoke. It was detected using mass spectrometry, and easily dissolves in water, posing a health risk to the lungs.[5]
Isomers: Cyanic acid and fulminic acid
Low-temperature photolysis of solids containing HNCO has been shown to make H-O-C≡N, known as cyanic acid or hydrogen cyanate; it is a tautomer of isocyanic acid.[6] Pure cyanic acid has not been isolated, and isocyanic acid is the predominant form in all solvents.[4] Note that sometimes information presented for cyanic acid in reference books is actually for isocyanic acid.Cyanic and isocyanic acids are isomers of fulminic acid (H-C=N-O), an unstable compound.[7]
See also
References
- Kurzer, Frederick (2000). "Fulminic Acid in the History of Organic Chemistry". Journal of Chemical Education 77 (7): 851–857. Bibcode:2000JChEd..77..851K. doi:10.1021/ed077p851.
Further reading
- Handbook of Chemistry and Physics, 65th. Edition, CRC Press (1984)
External links
- Walter, Wolfgang (1997). Organic Chemistry: A Comprehensive Degree Text and Source Book. Chichester: Albion Publishing. p. 364. ISBN 978-1-898563-37-2. Retrieved 2008-06-21.
- Cyanic acid from NIST Chemistry WebBook (accessed 2006-09-09)
|
||
|
||
|
No comments:
Post a Comment