Though I wasn't an egghead in Public School I did love science a lot and always got my best grades in science and I also read religiously science fiction by Isaac Asimov like "I, Robot" and Robert Heinlein "Stranger in a Strange Land" from the time I was about 10 years old and sophisticated enough to read stuff like this.
But, my favorite science experiment had to be when Mr. Addison, my junior High Science Teacher brought this glassware setup with a DC Battery where he put water in this thing and off of one pole of the DC battery came Hydrogen and off the other pole came Oxygen. What he had done was turn water right in front of my eyes into Hydrogen and Oxygen which was amazing. Both the Hydrogen and Oxygen are very volatile too. The hydrogen (remember the Hindenburg?) and Oxygen (increases burning exponentially if something catches fire).
So, there were some risks to even conducting this experiment which made it all the more interesting to watch in person.
For example, you could take a 12 volt or 24 volt or most sizes of DC Batteries and take a lead off of one and conduct this experiment yourselves. However, it is also dangerous to do this so you don't want to die doing something like this with a car battery for example, because you might get shocked and not be able to get loose from it and have a heart attack and die if you put your lead underwater when it is bare with your bare hand on metal or even in the water at all. In other words you likely want to be wearing rubber gloves to insulate yourselves and never touch the water ever also even with your gloves or any other part oof your body.
begin quote from:
https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis#:~:text=Electrolysis%20is%20the%20process%20of,a%20unit%20called%20an%20electrolyzer.
Electrolysis is a promising option for hydrogen production from renewable resources. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. This reaction takes place in a unit called an electrolyzer. Electrolyzers can range in size from small, appliance-size equipment that is well-suited for small-scale distributed hydrogen production to large-scale, central production facilities that could be tied directly to renewable or other non-greenhouse-gas-emitting forms of electricity production.
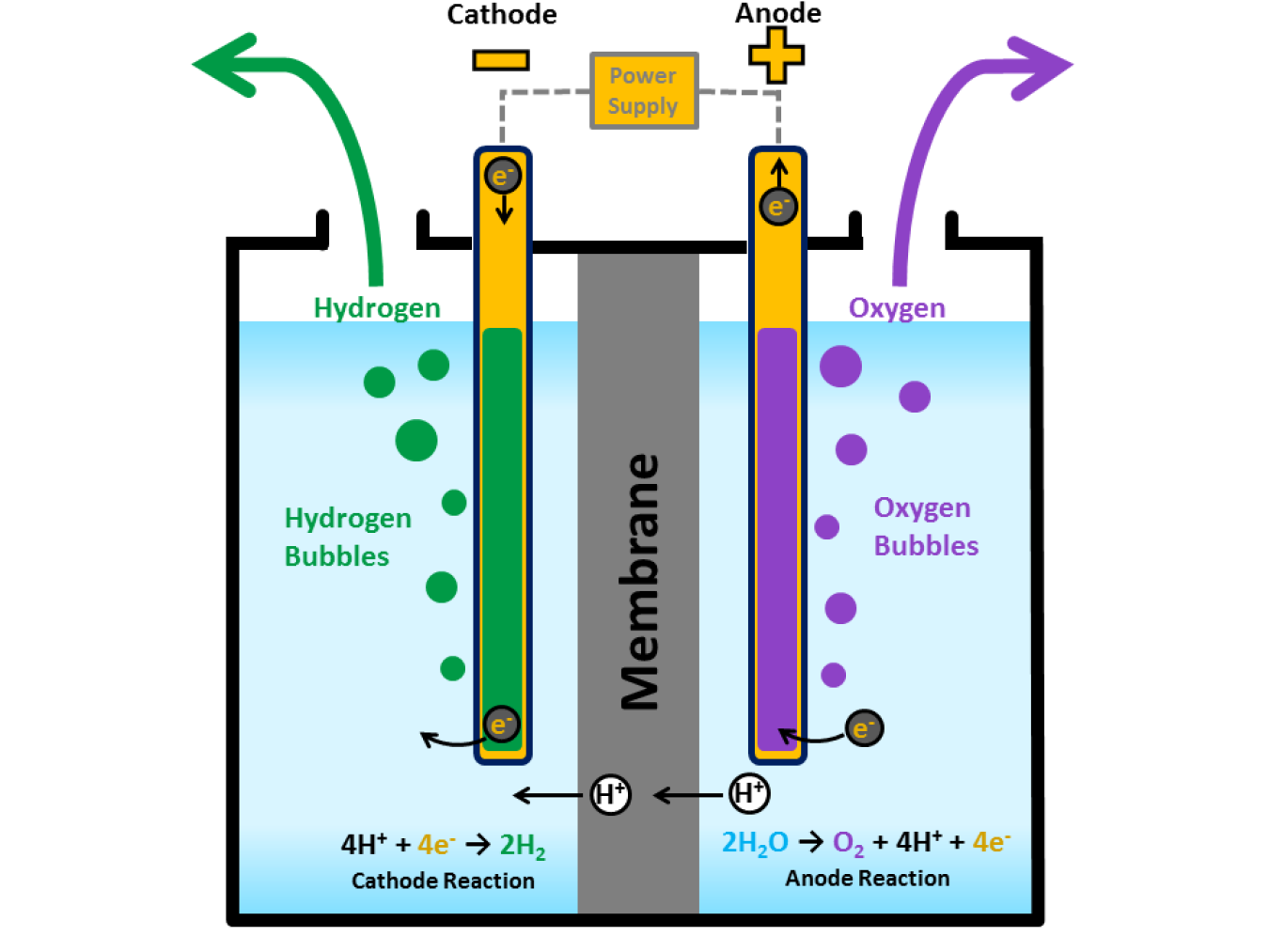
How Does it Work?
Like fuel cells, electrolyzers consist of an anode and a cathode separated by an electrolyte. Different electrolyzers function in slightly different ways, mainly due to the different type of electrolyte material involved.
POLYMER ELECTROLYTE MEMBRANE ELECTROLYZERS
In a polymer electrolyte membrane (PEM) electrolyzer, the electrolyte is a solid specialty plastic material.
- Water reacts at the anode to form oxygen and positively charged hydrogen ions (protons).
- The electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode.
- At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas. Anode Reaction: 2H2O → O2 + 4H+ + 4e- Cathode Reaction: 4H+ + 4e- → 2H2
ALKALINE ELECTROLYZERS
Alkaline electrolyzers operate via transport of hydroxide ions (OH-) through the electrolyte from the cathode to the anode with hydrogen being generated on the cathode side. Electrolyzers using a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte have been commercially available for many years. Newer approaches using solid alkaline exchange membranes as the electrolyte are showing promise on the lab scale.
SOLID OXIDE ELECTROLYZERS
Solid oxide electrolyzers, which use a solid ceramic material as the electrolyte that selectively conducts negatively charged oxygen ions (O2-) at elevated temperatures, generate hydrogen in a slightly different way.
- Water at the cathode combines with electrons from the external circuit to form hydrogen gas and negatively charged oxygen ions.
- The oxygen ions pass through the solid ceramic membrane and react at the anode to form oxygen gas and generate electrons for the external circuit.
Solid oxide electrolyzers must operate at temperatures high enough for the solid oxide membranes to function properly (about 700°–800°C, compared to PEM electrolyzers, which operate at 70°–90°C, and commercial alkaline electrolyzers, which operate at 100°–150°C). The solid oxide electrolyzers can effectively use heat available at these elevated temperatures (from various sources, including nuclear energy) to decrease the amount of electrical energy needed to produce hydrogen from water.
Why Is This Pathway Being Considered?
Hydrogen produced via electrolysis can result in zero greenhouse gas emissions, depending on the source of the electricity used. The source of the required electricity—including its cost and efficiency, as well as emissions resulting from electricity generation—must be considered when evaluating the benefits and economic viability of hydrogen production via electrolysis. In many regions of the country, today's power grid is not ideal for providing the electricity required for electrolysis because of the greenhouse gases released and the amount of fuel required due to the low efficiency of the electricity generation process. Hydrogen production via electrolysis is being pursued for renewable (wind) and nuclear energy options. These pathways result in virtually zero greenhouse gas and criteria pollutant emissions.
Potential for synergy with renewable energy power generation
Hydrogen production via electrolysis may offer opportunities for synergy with variable power generation, which is characteristic of some renewable energy technologies. For example, though the cost of wind power has continued to drop, the inherent variability of wind is an impediment to the effective use of wind power. Hydrogen fuel and electric power generation could be integrated at a wind farm, allowing flexibility to shift production to best match resource availability with system operational needs and market factors. Also, in times of excess electricity production from wind farms, instead of curtailing the electricity as is commonly done, it is possible to use this excess electricity to produce hydrogen through electrolysis.
It is important to note...
- Today's grid electricity is not the ideal source of electricity for electrolysis because most of the electricity is generated using technologies that result in greenhouse gas emissions and are energy intensive. Electricity generation using renewable or nuclear energy technologies, either separate from the grid, or as a growing portion of the grid mix, is a possible option to overcome these limitations for hydrogen production via electrolysis.
- The U.S. Department of Energy and others continue efforts to bring down the cost of renewable-based electricity production and develop more efficient coal-based electricity production with carbon capture, utilization, and storage. Wind-based electricity production, for example, is growing rapidly in the United States and globally.
Research Focuses On Overcoming Challenges
- Reducing the capital cost of the electrolyzer unit and the balance of the system, and improving energy efficiency for converting electricity to hydrogen.
- Integrating compression into the electrolyzer to avoid the cost of a separate hydrogen compressor needed to increase pressure for hydrogen storage.
No comments:
Post a Comment