end partial quote from:
Pangaea
Often when we think of Antarctica we think of only a frozen wastland most or all of the year with the ice and snow there never melting. This is true. But, it is also true that once Antarctica was no under ice and likely because of Global Warming and Global Climate change it will be free of ice eventually again (unless all this triggers another ice age like it often has in the past). However, then it might not be Antarctica that is frozen but other places instead. It sort of depends upon various different factors.
For example therapsid Lystrosaurus are found alongside of Glossopteris Flora.
begin quote from:
therapsid
Therapsid
From Wikipedia, the free encyclopedia
| Therapsids Temporal range: Early Permian–Holocene 275–0 Ma (Range includes mammals) |
|
|---|---|
 |
|
| Mounted skeleton of Inostrancevia alexandri, a gorgonopsian therapsid | |
| Scientific classification |
|
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Synapsida |
| Clade: | Sphenacodontoidea |
| Order: | Therapsida Broom, 1905 |
| Clades | |
Contents
Characteristics
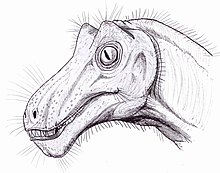
Illustration of Pristerognathus, a cat-sized therocephalian therapsid
Legs and feet
Therapsids had legs positioned more vertically beneath their bodies than were the sprawling legs of reptiles and pelycosaurs. Also compared to these groups, the feet were more symmetrical, with the first and last toes short and the middle toes long, an indication that the foot's axis was placed parallel to that of the animal, not sprawling out sideways. This orientation would have given a more mammal-like gait than the lizard-like gait of the pelycosaurs.[5]Jaw and teeth
Therapsids' temporal fenestrae are greater than those of the pelycosaurs. The jaws of some therapsids are more complex and powerful, and the teeth are differentiated into frontal incisors for nipping, great lateral canines for puncturing and tearing, and molars for shearing and chopping food.Fur and Endorthermy
Several characteritics in therapsids have been noted as being consistent with the development of endothermy: the presence of turbinates, erect limbs, highly vascularised bones, limb and tail proportions conductive to the preservation of body heat and the absence of growth rings in bones.[6] Therefore, like modern mammals, non-mammalian therapsids were most likely warm-blooded.Recent studies on Permian coprolites showcase that hair was present in at least some therapsids.[7] Hair is by any means present in the docodont Castorocauda, and whiskers are inferred from therocephalians and cynodonts.
Evolutionary history
See also: Evolution of mammals
| This section includes a list of references, but its sources remain unclear because it has insufficient inline citations. (December 2013) |
Raranimus, a primitive therapsid
Like all land animals, the therapsids were seriously affected by the Permian–Triassic extinction event; the very successful gorgonopsians dying out altogether and the remaining groups - dicynodonts, therocephalians, and cynodonts - reduced to a handful of species each by the earliest Triassic. The dicynodonts, now represented by a single family of large stocky herbivores, the Kannemeyeridae, and the medium-sized cynodonts (including both carnivorous and herbivorous forms), flourished worldwide throughout the Early and Middle Triassic. They disappear from the fossil record across much of Pangea at the end of the Carnian (Late Triassic), although they continued for some time longer in the wet equatorial band and the south.
Some exceptions were the still further derived eucynodonts. At least three groups of them survived. They all appeared in the Late Triassic period. The extremely mammal-like family, Tritylodontidae, survived into the Early Cretaceous. Another extremely mammal-like family, Tritheledontidae, are unknown later than the Early Jurassic. Mammaliaformes was the third group, including Morganucodon and similar animals. Many taxonomists refer to these animals as "mammals", though some limit the term to the mammalian crown group.
The non-eucynodont cynodonts survived the Permian-Triassic extinction; Thrinaxodon, Galesaurus and Platycraniellus are known from the Early Triassic. By the Middle Triassic, however, only the eucynodonts remained.
The therocephalians, relatives of the cynodonts, managed to survive the Permian-Triassic extinction and continued to diversify through the Early Triassic period. Approaching the end of the period, however, the therocephalians were in decline to eventual extinction, likely outcompeted by the rapidly diversifying Saurian lineage of diapsids, equipped with sophisticated respiratory systems better suited to the very hot, dry and oxygen-poor world of the End-Triassic.
Dicynodonts were long thought to have become extinct near the end of the Triassic, but there is evidence that they survived into the Cretaceous. Their fossils have been found in Gondwana.[10] This is an example of Lazarus taxon. Other animals that were common in the Triassic also took refuge here, such as the temnospondyls.
Mammals are the only living therapsids. The mammalian crown group, which evolved in the Early Jurassic period, radiated from a group of mammaliaforms that included the docodonts. The mammaliaforms themselves evolved from probainognathians, a lineage of the eucynodont suborder.
Taxonomy
Classification
Anteosaurus, an anteosaur
- Class Synapsida
- ORDER THERAPSIDA *
- ?Family † Tetraceratopsidae
- Suborder † Biarmosuchia *
- Family † Biarmosuchidae
- Family † Eotitanosuchidae
- Eutherapsida
- Suborder † Dinocephalia
- Family † Estemmenosuchidae
- ?Infraorder † Anteosauria
- Family † Anteosauridae
- Family † Brithopodidae
- Family † Deuterosauridae
- Family † Syodontidae
- ?Family † Stenocybidae
- † Tapinocephalia
- Family † Styracocephalidae
- Family † Tapinocephalidae
- Family † Titanosuchidae
- Neotherapsida
- Suborder † Anomodontia *
- Superfamily † Venyukoviamorpha
- Family † Otsheridae
- Family † Venyukoviidae
- Infraorder † Dromasauria
- Family † Galeopidae
- Infraorder † Dicynodonta
- Family † Endothiodontidae
- Family † Eodicynodontidae
- Family † Kingoriidae
- (Unranked) † Diictodontia
- Superfamily † Emydopoidea
- Family † Cistecephalidae
- Family † Emydopidae
- Superfamily † Robertoidea
- Family † Diictodontidae
- Family † Robertiidae
- Superfamily † Emydopoidea
- (Unranked) † Pristerodontia
- Family † Aulacocephalodontidae
- Family † Dicynodontidae
- Family † Kannemeyeriidae
- Family † Lystrosauridae
- Family † Oudenodontidae
- Family † Pristerodontidae
- Family † Shanisiodontidae
- Family † Stahleckeriidae
- Superfamily † Venyukoviamorpha
- Theriodontia *
- Suborder † Gorgonopsia
- Family † Gorgonopsidae
- Eutheriodontia
- Suborder † Therocephalia
- Family † Lycosuchidae
- (Unranked) † Scylacosauria
- Family † Scylacosauridae
- Infraorder †Eutherocephalia
- Family † Hofmeyriidae
- Family † Moschorhinidae
- Family † Whaitsiidae
- Superfamily Baurioidea
- Family † Bauriidae
- Family † Ericiolacteridae
- Family † Ictidosuchidae
- Family † Ictidosuchopsidae
- Family † Lycideopsidae
- Suborder Cynodontia *
- Family † Dviniidae
- Family † Procynosuchidae
- (Unranked) Epicynodontia
- Family † Galesauridae
- Family † Thrinaxodontidae
- Infraorder Eucynodontia
- (Unranked) † Cynognathia
- Family † Cynognathidae
- Family † Diademodontidae
- Family † Traversodontidae
- Family † Trirachodontidae
- Family † Tritylodontidae
- (Unranked) Probainognathia
- Family † Chinquodontidae
- Family † Probainognathidae
- (Unranked) † Ictidosauria
- Family † Tritheledontidae
- (Unranked) Mammaliaformes
- Class Mammalia
- (Unranked) † Cynognathia
- Suborder † Therocephalia
- Suborder † Gorgonopsia
- Suborder † Anomodontia *
- Suborder † Dinocephalia
Phylogeny
| Synapsida |
|
||||||||||||||||||||||||||||||
See also
References
- Thulbord, Tony; Turner, Susan (2003). "The last dicynodont: an Australian Cretaceous relict" (PDF). Proceedings of the Royal Society of London B 270: 985–993. doi:10.1098/rspb.2002.2296.
Further reading
- Benton, M. J. (2004). Vertebrate Palaeontology, 3rd ed., Blackwell Science.
- Carroll, R. L. (1988). Vertebrate Paleontology & Evolution. W. H. Freeman & Company, New York.
- Kemp, T. S. (2005). The origin and evolution of mammals. Oxford University Press.
- Romer, A. S. (1966). Vertebrate Paleontology. University of Chicago Press, 1933; 3rd ed.
- Bennett, A. F., & Ruben, J. A. (1986). "The metabolic and thermoregulatory status of therapsids." In The ecology and biology of mammal-like reptiles. Smithsonian Institution Press, Washington, DC, 207-218.
External links
| Wikispecies has information related to: Therapsida |
- "Therapsida: Mammals and extinct relatives" Tree of Life
- "Therapsida: overview" Palaeos
|
||||||||||||||||||||||||||||||||||||||||||||||
Lystrosaurus
From Wikipedia, the free encyclopedia
| Lystrosaurus Temporal range: Late Permian–Early Triassic, 255–250 Ma |
|
|---|---|
 |
|
| Lystrosaurus hedini skeleton at the Museum of Paleontology, Tuebingen | |
| Scientific classification |
|
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Synapsida |
| Order: | Therapsida |
| Infraorder: | †Dicynodontia |
| Family: | †Lystrosauridae |
| Genus: | †Lystrosaurus Cope, 1870 |
| Species | |
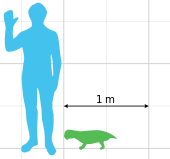
Being a dicynodont, Lystrosaurus had only two teeth, a pair of tusk-like canines, and is thought to have had a horny beak that was used for biting off pieces of vegetation. Lystrosaurus was a heavily built, herbivorous animal, approximately the size of a pig. The structure of its shoulders and hip joints suggests that Lystrosaurus moved with a semi-sprawling gait. The forelimbs were even more robust than the hindlimbs, and the animal is thought to have been a powerful digger that nested in burrows.
Lystrosaurus was by far the most common terrestrial vertebrate of the Early Triassic, accounting for as many as 95% of the total individuals in some fossil beds.[3] It has often been suggested that it had anatomical features that enabled it to adapt better than most animals to the atmospheric conditions that were created by the Permian–Triassic extinction event and which persisted through the Early Triassic—low concentrations of oxygen and high concentrations of carbon dioxide.[citation needed] However, recent research suggests that these features were no more pronounced in Lystrosaurus than in genera that perished in the extinction or in genera that survived but were much less abundant than Lystrosaurus.[citation needed]
Contents
Description
Size of Lystrosaurus murrayi relative to a human.
Unlike other therapsids, dicynodonts had very short snouts and no teeth except for the tusk-like upper canines. Dicynodonts are generally thought to have had horny beaks like those of turtles, for shearing off pieces of vegetation which were then ground on a horny secondary palate when the mouth was closed. The jaw joint was weak and moved backwards and forwards with a shearing action, instead of the more common sideways or up and down movements. It is thought that the jaw muscles were attached unusually far forward on the skull and took up a lot of space on the top and back of the skull. As a result, the eyes were set high and well forward on the skull, and the face was short.[5]
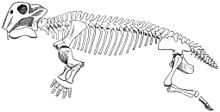
Features of the skeleton indicate that Lystrosaurus moved with a semi-sprawling gait. The lower rear corner of the scapula (shoulder blade) was strongly ossified (built of strong bone), which suggests that movement of the scapula contributed to the stride length of the forelimbs and reduced the sideways flexing of the body.[6] The five sacral vertebrae were massive but not fused to each other and to the pelvis, making the back more rigid and reducing sideways flexing while the animal was walking. Therapsids with fewer than five sacral vertebrae are thought to have had sprawling limbs, like those of modern lizards.[6] In dinosaurs and mammals, which have erect limbs, the sacral vertebrae are fused to each other and to the pelvis.[7] A buttress above each acetabulum (hip socket) is thought to have prevented dislocation of the femur (thigh bone) while Lystrosaurus was walking with a semi-sprawling gait.[6] The forelimbs of Lystrosaurus were massive,[6] and Lystrosaurus is thought to have been a powerful burrower.[8]
Distribution and species
Lystrosaurus fossils have been found in many Late Permian and Early Triassic terrestrial bone beds, most abundantly in Africa, and to a lesser extent in parts of what are now India, China, Mongolia, European Russia, and Antarctica (which was not over the South Pole at the time).[6]Species found in Africa
Lystrosaurus murrayi
L. maccaigi is the largest and apparently most specialized species, while L. curvatus was the least specialized. A Lystrosaurus-like fossil, Kwazulusaurus shakai, has also been found in South Africa. Although not assigned to the same genus, K. shakai is very similar to L. curvatus. Some paleontologists have therefore proposed that K. shakai was possibly an ancestor of or closely related to the ancestors of L. curvatus, while L. maccaigi arose from a different lineage.[8] L. maccaigi is found only in sediments from the Permian period, and apparently did not survive the Permian–Triassic extinction event. Its specialized features and sudden appearance in the fossil record without an obvious ancestor may indicate that it immigrated into the Karoo from an area in which Late Permian sediments have not been found.[8]
L. curvatus is found in a relatively narrow band of sediments from shortly before and after the extinction, and can be used as an approximate marker for the boundary between the Permian and Triassic periods. A skull identified as L. curvatus has been found in late Permian sediments from Zambia. For many years it had been thought that there were no Permian specimens of L. curvatus in the Karoo, which led to suggestions that L. curvatus immigrated from Zambia into the Karoo. However, a re-examination of Permian specimens in the Karoo has identified some as L. curvatus, and there is no need to assume immigration.[8]
L. murrayi and L. declivis are found only in Triassic sediments.[8]
Lystrosaurus georgi
Other species
Lystrosaurus georgi fossils have been found in the Earliest Triassic sediments of the Moscow Basin in Russia. It was probably closely related to the African Lystrosaurus curvatus,[6] which is regarded as one of the least specialized species and has been found in very Late Permian and very Early Triassic sediments.[8]History
Geographical distribution of Lystrosaurus ( ) and contemporary fossils in Gondwana.
Plate tectonics
The discovery of Lystrosaurus fossils at Coalsack Bluff in the Transantarctic Mountains by Edwin H. Colbert and his team in 1969–70 helped confirm the theory of plate tectonics and convince the last of the doubters, since Lystrosaurus had already been found in the lower Triassic of southern Africa as well as in India and China.[12]Paleoecology
Dominance of the Early Triassic
Lystrosaurus is notable for dominating southern Pangaea during the Early Triassic for millions of years. At least one unidentified species of this genus survived the end-Permian mass extinction and, in the absence of predators and of herbivorous competitors, went on to thrive and re-radiate into a number of species within the genus,[13] becoming the most common group of terrestrial vertebrates during the Early Triassic; for a while 95% of land vertebrates were Lystrosaurus.[13][14] This is the only time that a single species or genus of land animal dominated the Earth to such a degree.[15] A few other Permian therapsid genera also survived the mass extinction and appear in Triassic rocks—the therocephalians Tetracynodon, Moschorhinus and Ictidosuchoides—but do not appear to have been abundant in the Triassic;[8] complete ecological recovery took 30 million years, spanning the Early and Middle Triassic.[16]Several attempts have been made to explain why Lystrosaurus survived the Permian–Triassic extinction event, the "mother of all mass extinctions",[17] and why it dominated Early Triassic fauna to such an unprecedented extent:
Lystrosaurus murrayi
- One of the more recent theories is that the Permian–Triassic extinction event reduced the atmosphere's oxygen content and increased its carbon dioxide content, so that many terrestrial species died out because they found breathing too difficult.[14] It has therefore been suggested that Lystrosaurus survived and became dominant because its burrowing life-style made it able to cope with an atmosphere of "stale air", and that specific features of its anatomy were part of this adaptation: a barrel chest that accommodated large lungs, short internal nostrils that facilitated rapid breathing, and high neural spines (projections on the dorsal side of the vertebrae) that gave greater leverage to the muscles that expanded and contracted its chest. However, there are weaknesses in all these points: the chest of Lystrosaurus was not significantly larger in proportion to its size than in other dicynodonts that became extinct; although Triassic dicynodonts appear to have had longer neural spines than their Permian counterparts, this feature may be related to posture, locomotion or even body size rather than respiratory efficiency; L. murrayi and L. declivis are much more abundant than other Early Triassic burrowers such as Procolophon or Thrinaxodon.[8]
Lystrosaurus skeletal diagram
- The suggestion that Lystrosaurus was helped to survive and dominate by being semi-aquatic has a similar weakness: although amphibians become more abundant in the Karoo's Triassic sediments, they were much less numerous than L. murrayi and L. declivis.[8]
- The most specialized and the largest animals are at higher risk in mass extinctions; this may explain why the unspecialized L. curvatus survived while the larger and more specialized L. maccaigi perished along with all the other large Permian herbivores and carnivores.[8] Although Lystrosaurus generally looks adapted to feed on plants similar to Dicroidium, which dominated the Early Triassic, the larger size of L. maccaigi may have forced it to rely on the larger members of the Glossopteris flora, which did not survive the end-Permian extinction.[8]
- Only the 1.5 metres (4.9 ft)–long therocephalian Moschorhinus and the large archosauriform Proterosuchus appear large enough to have preyed on the Triassic Lystrosaurus species, and this shortage of predators may have been responsible for a Lystrosaurus population boom in the Early Triassic.[8]
- Perhaps the survival of Lystrosaurus was simply a matter of luck.[13]
In popular culture
Fossil specimen, Staatliches Museum für Naturkunde Stuttgart
- BBC 2002 documentary The Day The Earth Nearly Died, a program which discuss the Permian extinction. In the program, the narrator says that Lystrosaurus was the only therapsid to survive the extinction, and that it was the ancestor to all mammals, even humans. This is not correct, as paleontologists do not regard dicynodonta as ancestral to mammals.
- Impossible Pictures production Walking with Monsters. Here, it was shown evolving from the little dicynodont Diictodon, even though both species lived at the same time though this may be a Triassic species of Lystrosaurus as most species died out in the Permian extinction. The program shows evolution of other creatures of the same time period.
- Animal Armageddon, 5th episode, "explaining" that the different Lystrosaurus species had interbred with each other to adapt better and to survive during the transition from Permian to Triassic.
- Lystrosaurus appeared in the Rite of Spring segment in the 1940 animated film Fantasia, where it is shown to dig out clams along with the Plateosaurus and it was one of the animals led by the Stegosaurus.
- Lystrosaurus was added to the dinosaur sandbox survival video game ARK: Survival Evolved in PC patch v240 on May 4, 2016.
See also
References
- Erwin DH (1993). The great Paleozoic crisis; Life and death in the Permian. Columbia University Press. ISBN 0-231-07467-0.
External links
- Palaeos.com: Dicynodontia
- Hugh Rance, The Present is the Key to the Past: "Mammal-like reptiles of Pangea"
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
begin quote from:
Glossopteris Flora (in Antarctica)
Glossopteris
From Wikipedia, the free encyclopedia
|
|
This article's lead section may not adequately summarize key points of its contents. (December 2015) |
| Glossopteris Temporal range: Permian |
|
|---|---|
 |
|
| Glossopteris sp. | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | †Pteridospermatophyta |
| Order: | †Glossopteridales |
| Family: | †Glossopteridaceae |
| Genus: | †Glossopteris |
| Species | |
|
|
 |
|
| Fossils of the gymnosperm Glossopteris (dark green) found in all of the southern continents provide strong evidence that the continents were once amalgamated into a supercontinent Gondwana | |
Contents
History
The Glossopteridales arose in the Southern Hemisphere around the beginning of the Permian Period (298.9 million years ago).[1] Their distribution across several, now detached, landmasses led Eduard Suess, amongst others, to propose that the southern continents were once amalgamated into a single supercontinent—Pangea.[2] These plants went on to become the dominant elements of the southern flora through the rest of the Permian but disappeared in almost all places at the end of the Permian (252.17 million years ago).[3][4][5] The only convincing Triassic records are very earliest Triassic leaves from Nidpur, India,[6] but even these records are somewhat questionable owing to faulting and complex juxtapositioning of Permian and Triassic strata at Nidpur. Although most modern palaeobotany textbooks cite the continuation of glossopterids into later parts of the Triassic and, in some cases into the Jurassic, these ranges are erroneous and are based on misidentification of morphologically similar leaves such as Gontriglossa,[7] Sagenopteris, or Mexiglossa.[8] Glossopterids were, thus, one of the major casualties of the end-Permian mass extinction event.[9]
Location of Glossopteris remains shown in green in the former Supercontinent Gondwana.
Taxonomy
Long considered a fern after its discovery in the 1820s,[21] it was later assigned to the gymnosperms. The genus is placed in the division Pteridospermatophyta. In reality, many of the plant groups included within this division are only distantly related to one another. Glossopterids' relationships with other groups remain obscure. Most recent phylogenetic analyses favour placement of glossopterids as sister to a large group including Corystospermales, Caytoniales, Bennettitales, Pentoxylales, Gnetales (in some analyses), and angiosperms.[22] A few analyses favour alternative links with Ginkgoales, Cordaitales and Pinales.Glossopteris should strictly be used to refer to the distinctive spathulate fossil leaves with reticulate venation, however, the term has also been used to refer to the parent plant as a whole.[23]
Description
Glossopteris was a woody, seed-bearing shrub or tree, some apparently reaching 30 metres (98 ft) tall. They had a softwood interior that resembles conifers of the family Araucariaceae.[24] Seeds were borne on one side of variably branched or fused structures,[25][26][27][28][29][30] and microsporangia containing pollen were borne in clusters at the tips of slender filaments.[31] Both the seed- and pollen-bearing organs were partially fused (adnate) to the leaves, or, in some cases, possibly positioned in the axils of leaves. The homologies of the flattened seed-bearing structures have remained particularly controversial with some arguing that the fertile organs represent megasporophylls (fertile leaves) whereas others have interpreted the structures as flattened, seed-bearing, axillary axes (cladodes). It is unclear whether glossopterids were monoecious or dioecious.Paleoecology
They are interpreted to have grown in very wet soil conditions,[32][33] similar to the modern Bald Cypress. The leaves ranged from about 2 cm to over 30 cm in length.The profile of glossopterid trees is largely speculative as complete trees have not been preserved. However, based on analogies with modern high-latitude plants Glossopteris trees probably tapered upwards like a Christmas tree and were relatively widely spaced to take advantage of the low-angle sunlight at high latitudes. Instead of needles, they had large, broad lance- or tongue-shaped leaves that fell to the ground at the end of summer. The fossil leaves are commonly found as dense accumulations representing autumnal leaf banks.[34][35] The broad fossilized growth rings in many Glossopteris woods reveal that the plants experienced strong growth spurts each spring-summer but underwent abrupt cessation of growth before each following winter.[36]
Glossopteris leaves are morphologically simple so there are few characters that can be used to differentiate species.[37] Consequently, many past researchers have considered the Permian Glossopteris flora to be rather homogeneous with the same species distributed throughout the Southern Hemisphere. However, more recent studies of the more morphologically diverse fertile organs have shown that taxa had more restricted regional distributions and several intra-gondwanan floristic provinces are recognizable. Seeds, much too large to be wind-borne, could not have blown across thousands of miles of open sea, nor is it likely they have floated across vast oceans. Observations such as these led the Austrian geologist Eduard Suess to deduce that there had once been a land bridge between these areas. He named this large land mass Gondwanaland (named after the district in India where the plant Glossopteris was found). These same observations would also lend support to Alfred Wegener's Continental drift theory.
The first Antarctic specimens of Glossopteris were discovered by members of Robert Scott's doomed Terra Nova Expedition. The expedition members abandoned much of their gear in an effort to reduce their load, but kept 35 pounds of Glossopteris fossils; these were found alongside their bodies.[38]
Outcrops in Brazil
The first investigation of a Glossopteris flora associated with coal seams within a paleogeographic and palaeoclimatic context, in the Paraná Basin, southern Brazil, was that by geologist Israel Charles White in 1908. This allowed correlation between Gondwanan coal deposits in southern Brazil and those documented in South Africa, Australia, India and Antarctica, and showed that this flora flourished in latitudes near the south pole.In Rio Grande do Sul, Glossopteris leaves were found in paleorrota at Mina Faxinal, in Arroio dos Ratos at Mina Morro do Papaléo in Mariana Pimentel and Quitéria in Pantano Grande. Various species were recovered from the Rio Bonito Formation at these sites including G. angustifolia, G. brasiliensis, G. browniana, G. communis, G. indica and G. occidentalis.[39]
References
- Adami-Rodrigues, Karen; Alves de Souza, Paulo; Iannuzzi, Roberto; Pinto, Irajá Damiani (2004). "Herbivoria em Floras Gonduânicas do NeoPaleózoico do Rio Grande do Sul" (PDF). Revista Brasileira de Paleontologia 7 (2): 93–102.
Sources
- Brongniart, A. 1828. Prodrome d’une histoire des végétaux fossiles. Paris. 223 pp.
- Brongniart, A. 1832. Histoire des végétaux fossiles ou recherches botaniques et géologiques sur les végétaux renfermés dans les diverses couches du globe. G. Dufour and E. D’Ocagne, Paris 1: 265-288.
- Anderson, H.M. & Anderson, J.M. 1985. The Palaeoflora of Southern Africa: Prodromus of Southern African Megafloras, Devonian to Lower Cretaceous. A.A. Balkema, Rotterdam. 416 pp.
- Chandra, S. & Surange, K.R. 1979. Revision of the Indian species of Glossopteris. Monograph 2. Birbal Sahni Institute of Palaeobotany, Lucknow. 301 pp.
- Davis, Paul and Kenrick, Paul. 2004. Fossil Plants. Smithsonian Books (in association with the Natural History Museum of London), Washington, D.C. ISBN 1-58834-156-9
- Gould, R. E. and Delevoryas, T., 1977. The biology of Glossopteris: evidence from petrified seed-bearing and pollen-bearing organs. Alcheringa, 1: 387-399.
- Pant DD 1977 The plant of Glossopteris. J Indian Bot Soc 56: 1-23.
- Pant, D.D. & Gupta, K.L. 1971. Cuticular structure of some Indian Lower Gondwana species of Glossopteris Brongniart. Part 2. - Palaeontographica, 132B: 130-152.
- Pant, D.D. & Nautiyal, D.D. 1987. Diphyllopteris verticellata Srivastava, the probable seedling of Glossopteris from the Paleozoic of India. - Rev. Palaeobot. Palynol., 51: 31-36.
- Pant, D.D. & Pant, R. 1987. Some Glossopteris leaves from Indian Triassic beds. - Palaeontographica, 205B: 165-178.
- Pant, D.D. & Singh, K.B. 1971. Cuticular structure of some Indian Lower Gondwana species of Glossopteris Brongniart. Part 3. - Palaeontographica, 135B: 1-40.
- Pigg, K. B. 1990. Anatomically preserved Glossopteris foliage from the central Transantarctic Mountains. Rev. Palaeobot. Palynol. 66: 105-127.
- Pigg, K.B. & McLoughlin, S. 1997. Anatomically preserved Glossopteris leaves from the Bowen and Sydney basins, Australia. Review of Palaeobotany and Palynology, 97: 339-359.
- Plumstead, E.P. (1969), Three thousand million years of plant life in Africa. Alex L. du Toit Memorial Lecture no. 11. Trans. Geol. Soc. S. Afr. 72 (annex.): 1-72.
- Taylor, E.L, Taylor, T.N. & Collinson, J.W. 1989. Depositional setting and palaeobotany of Permian and Triassic permineralized peat from the central Transantarctic Mountains, Antarctica. - Internat. J. Coal Geol., 12: 657-679.
External links
| Wikimedia Commons has media related to Glossopteris. |















No comments:
Post a Comment